Symbol Nomenclature for Glycans (SNFG)
Created: October 15, 2015; Last updated: June 27, 2022
Standardization in drawing glycan structures is essential for efficient communication. The tools and methodology illustrated here have become widely accepted by the scientific community. Use of these symbols to represent glycans is now strongly recommended for all scientific manuscripts and other publications. The following are pages with additional information about the Symbol Nomenclature For Glycans (SNFG):
Organizations and Publications Adopting SNFG
Software tools supporting the SNFG
Citation:
- Symbol Nomenclature for Graphical Representation of Glycans, Glycobiology 25: 1323-1324, 2015. Citation link (PMID 26543186).
- Updates to the Symbol Nomenclature for Glycans guidelines, Glycobiology 29:620-624, 2019. Citation link (PMID 31184695).
Page Content
- SNFG rendering programs
- Symbol Nomenclature
- Examples of Glycan Symbol Nomenclature
- CMYK and RGB color assignments
- Substituent list and chemical structures
- Monosaccharide abbreviations and names
- Updates to SNFG versions
SNFG rendering programs ☝
- 3D-Symbol Nomenclature for Glycans (3D-SNFG): Create 3D atomic models of glycans
Direct weblink | Citation link. - DrawGlycan SNFG: Convert IUPAC input strings to sketches of glycans and glycopeptides
Direct weblink | App download | Citation link. - GlycanBuilder2-SNFG: GlycanBuilder updated to handle SNFG
App download | Citation link.
Symbol Nomenclature ☝
Downloadable files: Drawing format | Presentation/Slide format
Each symbol represents a specific monosaccharide or class of monosaccharides found in nature. Hover over the symbol with pointer to see the full monosaccharide name. Click on a symbol to link to the corresponding PubChem entry. Symbols can also be copied with embedded links from the table using right/control-click or highlight-copy (highlight a symbol, then control-c [on pc], or command⌘-c [on mac]). However links may not copy in some browsers. Symbols with embedded PubChem URLs are therefore also available in the presentation/slide format attachments (see links above the table). A high-quality SVG object file is also provided.
Table 1. Monosaccharide symbol nomenclature
Notes
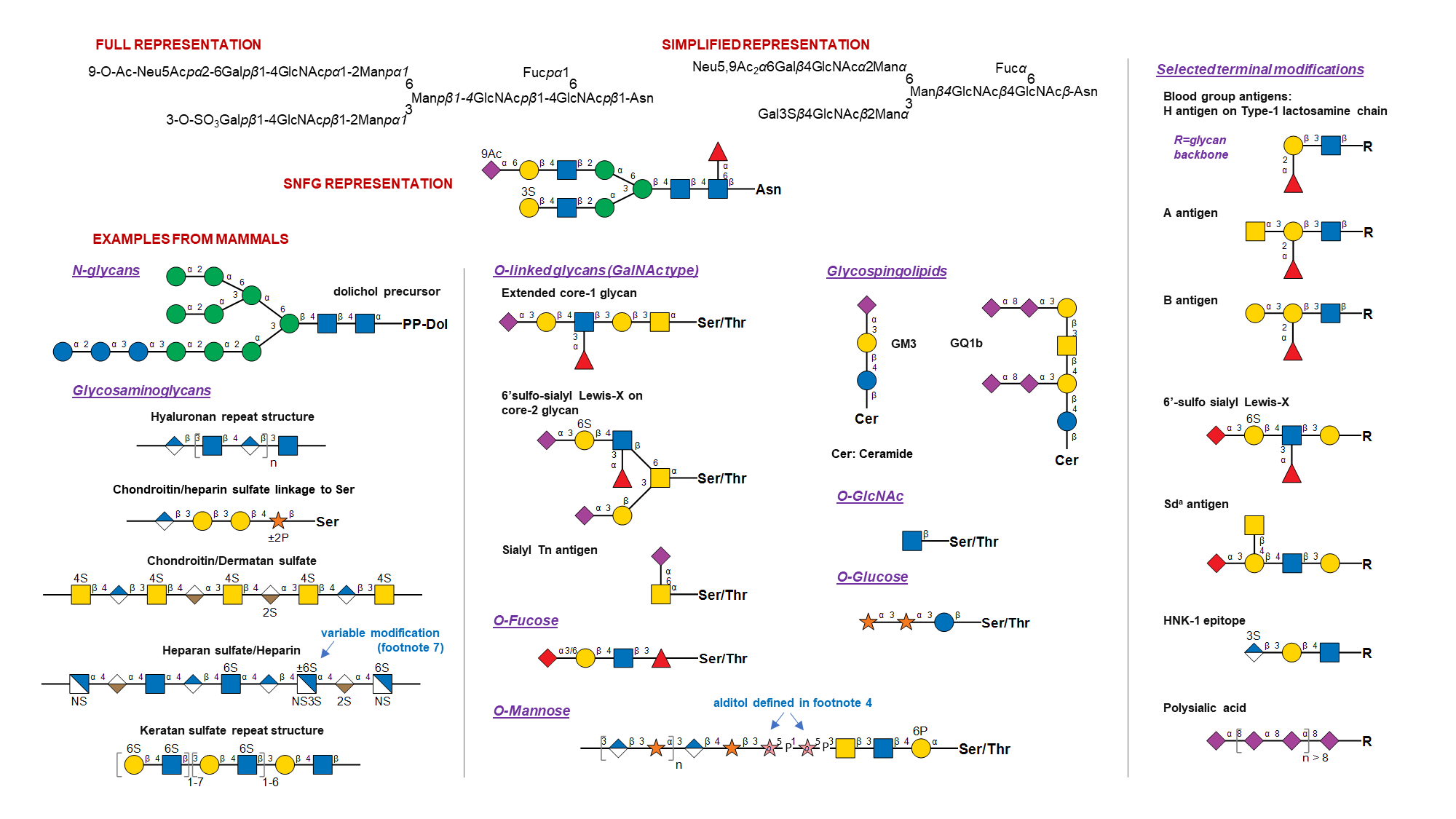
- General: The monosaccharide symbols presented here are from the Fourth Edition of the Essentials of Glycobiology. They are extended from the symbols in the Second Edition to cover a wider range of monosaccharides found in nature. While previous versions allowed conversion of monosaccharide symbols to black and white representation, this is no longer possible. A listing of abbreviated, full, and complete names of all assigned monosaccharides is provided in Table 4. Selected examples depicting SNFG usage are presented below, for various living organisms.
- Drawing recommendations: All downloadable symbols follow CMYK colors as shown in Table 2, which were generated in Adobe Illustrator. Recommended CMYK to RGB conversion is also provided. While there is no hard rule, glycans are typically sketched to orient their non-reducing end in either the left or upward direction.
- Shape, color and symbol orientation: Shapes and colors are completely consistent with stereochemistry only for hexoses, hexosamines, N-acetylhexosamines, hexuronic acids, and pentoses. Shapes only are consistent for deoxyhexoses, deoxy-N-acetylhexosamines, dideoxyhexoses, and nonulosonic acids. Avoid rotating the symbols.
- Ring configuration: A colored symbol encodes a defined monosaccharide (including D or L) independent of rotation or mirroring. Pyranose form is assumed by default for all monosaccharides except Api. Symbols used at the reducing end without specification of α/β represent all forms that a monosaccharide can adopt due to mutarotation. A few monosaccharides have absolute configurations implicitly specified in their name (D for Abe, Bac, Dha, Kdo, Mur, Par, Tyv; L for Col; DD for Kdn, Neu, Leg, 4eLeg; LL for Pse, Aci; LD for 8eLeg; DL for 8eAci). For all other residues, absolute configuration is assumed by default: L for Ara, Fuc, Ido, IdoA, Rha, Alt, AltA, Sor, Api; and D for other monosaccharides.
Less common configurations need to be stated in a figure legend or by adding letters inside the symbol (e.g., adding D or L to the symbol). Epimers at C8 of nonulosonic acids can be indicated by adding "8D" or "8L" inside the symbol (8L for 8eLeg, 8D for 8eAci). Furanose rings can be indicated by adding an italicized "f", alditols indicated with an italicized "o", and open ring at the reducing end using "a" inside the symbol. Other modifications of the monosaccharide base stereochemistry can be specified using two letters within the monosaccharide symbol: "en" for didehydro, "an" for anhydro, "on" for lactone and "am" for lactam. The default carbon positions for these modifications in sialic acids are at 2-3 for en, 2-7 for an, 1-7 for on, and 1-5 for am. Departures from this default should be specified in figure footnotes/legends. - Linkage presentation: Anomeric notation and destination linkage can be indicated in IUPAC style in figures, with or without a dash and with or without the originating carbon number (but with no commas or spacing) (e.g., Neu5Acα2-3Galβ1-4GlcNAc, Neu5Acα3Galβ4GlcNAc, or equivalents in symbol drawings).
All monosaccharide glycosidic linkages are assumed to originate from C-1, except for 2-ketoses, which are assumed to be linked from C-2. Linkages in the schematics should be sorted to appear in the clockwise order, with the linkages to the lowest carbon number occurring to the left. Optionally, these may also follow the Oxford System that embeds both the specificity and anomericity of the monosaccharide linkages. Dual linkages (e.g., an outgoing linkage from an aldose in open form) can be shown by double lines. Linkages involving carbon-carbon bond (e.g., in C-glycosides) can be shown in different color. An internal phosphodiester can be shown with -P- between the symbols for the linked monosaccharides, with linkage positions specified. - Nonulosonic acids (including sialic acids): Specific symbols are provided for the core sialic acids (3-deoxy-non-2-ulosonic acids / 3-deoxy-nonulosonic acids): Neu, Neu5Ac, Neu5Gc, and Kdn. Symbols are also available for the core sialic-acid like 3,9-dideoxy-non-2-ulosonic acids / 3,9-dideoxy-nonulosonic acids: pseudaminic acid (Pse), legionaminic acid (Leg), acinetaminic acid (Aci) and 4-epi-legionaminic acid (4eLeg). Modifications to these monosaccharides may be indicated in diagrams (e.g., 9Ac for 9-O-acetylation). A red diamond can be used for any Sia (sialic acid, type unknown, whether Neu5Gc or Neu5Ac, or any of the other >90 forms known to date). The NulO concept page provides a detailed catalog of currently known NulOs, primary citations and related database links.
- Carbohydrate modifications:
Modification of any base monosaccharide by chemical substituent is described using a number indicating the linkage position
and text indicating the substituent-type, e.g., 3S for 3-O-sulfate, 6P for 6-O-phosphate, and
4,6Pyr for 4,6-pyruvyl [4,6-O-(1-carboxyethylidene)]. The abbreviated text used to describe common substituents is listed in Table 3,
along with associated chemical drawings. The following guidelines are used to describe monosaccharide substituents:
i) Substituent names and abbreviations are guided by IUPAC convention, but common names are also allowed to keep with usage in the field and to enhance human readability.
ii) Substituent abbreviations are ideally 2-3 letters long. To save space, subscripts maybe dropped, e.g. Kdn4,7Ac2 is pictorially depicted as a green diamond with 4,7Ac rather than 4,7Ac2.
iii) Avoid numbers when describing substituents if possible, in order to reduce confusion with number used to depict linkage position. If numbers are necessary, then the substituent should be enclosed within parenthesis.
iv) Substituents should appear in alphabetical order based on Table 3. If the same modification appears at multiple carbon-locations this should be presented in numerical order.
v) The attachment of a substituent to the hydroxyl group on the base monosaccharide would result in an O-linked substituent and similar modification on amine would indicate N-linked addition. Thus, Neu4,5Ac28Me indicates O-acetylation at C4 of Neu (neuraminic acid), N-acetyl at C5, and O-methyl at C8.
vi). Substituents may be concatenated to indicate multiple modifications, with position indicated for the first substituent, e.g. 'Neu5(Gc2Ac)' depicting the C5 modification of Neu by a glycolyl group which is further modified by O-acetyl at C2 of the glycolyl substituent. If a given string concatenation results in a non-unique chemical description, the exact chemical structure should be provided using IUPAC rules.
vii) The presence of variable amounts of substituents can be indicated using +/- symbol or by indicating % presence if known, e.g. "60% 3Ac" to indicate presence of 3Ac on 60% of a residue or repeating unit.
viii) Substituents not in Table 3 may be included using above guidelines with footnotes describing the entity type/chemistry. A symbol (like *, † or ‡) above the SNFG monosaccharide and associated footnote may also be used when text describing substituent becomes large. - Amino substitution: The position for N-substitution is assumed in the base symbol to be at the most common site(s), for example the 2-position of glucosamine, the 5-position of neuraminic acid, and the 5 & 7 positions of pseudaminic acid. By default, it is assumed that these N-substituted groups are modified, e.g., NS for N-sulfate group on glucosamine is assumed to be at the 2-position. For amino sugars in which the nitrogen is not at the most common carbon, add a number to the N (e.g., Rha4N is shown as a green triangle with 4N attached). Additionally, atypical acetamido groups may be represented using NAc (e.g. Fuc4NAc is shown using red triangle with 4NAc attached).
- Ambiguous linkage position and glycan mixtures: Brackets (either straight or curly) indicate attachment of specific monosaccharides or structures to any residue within the glycan. Restrictions on the nature of attachment are specified by including linkage data on the bond outside the brackets. Constraints on attachment site are shown using asterisk followed by a number (*#), specified both on the bond and its attachment site(s). Such renderings are used to indicate ambiguity in the structure of a specific glycan. By extension, glycan mixtures may be depicted by including [number range] along the bond as shown in the examples.
- Ambiguous monosaccharides: White symbols based on the standard shapes designate monosaccharides with unknown/undefined stereochemistry (e.g., a white circle designates a hexose, type not defined, or a white diamond, any deoxynonulosonic acid). Other unknown or partially defined monosaccharides may be represented using a white flat hexagon.
- Structures not present in table and non-glycan assignments: Monosaccharides absent in Table 1 or modifications that cannot be represented using the above rules, may be indicated using a single non-italicized letter (A..Z) within an SNFG white symbol, with additional details provided in figure footnote or legends. The choice of white symbol to use should fit the generic type if possible: circle for hexose, triangle for deoxyhexose etc.; otherwise, the white pentagon should be used. Any black shape can be used to depict non-monosaccharide structures and detailed definitions should be provided in figure legends. More complicated modifications of the SNFG are discouraged, and if these modifications are made should not be referred to as following the SNFG nomenclature.
Examples of Glycan Symbol Nomenclature ☝
Table 2. CMYK and RGB color assignments ☝
| Color | CMYK settings | RGB settings |
|---|---|---|
| White | 0/0/0/0 | 255/255/255 |
| Blue | 100/50/0/0 | 0/114/188 |
| Green | 100/0/100/0 | 0/166/81 |
| Yellow | 0/15/100/0 | 255/212/0 |
| Light blue | 41/5/3/0 | 143/204/233 |
| Pink | 0/47/24/0 | 246/158/161 |
| Purple | 38/88/0/0 | 165/67/153 |
| Brown | 32/48/76/13 | 161/122/77 |
| Orange | 0/65/100/0 | 244/121/32 |
| Red | 0/100/100/0 | 237/28/36 |
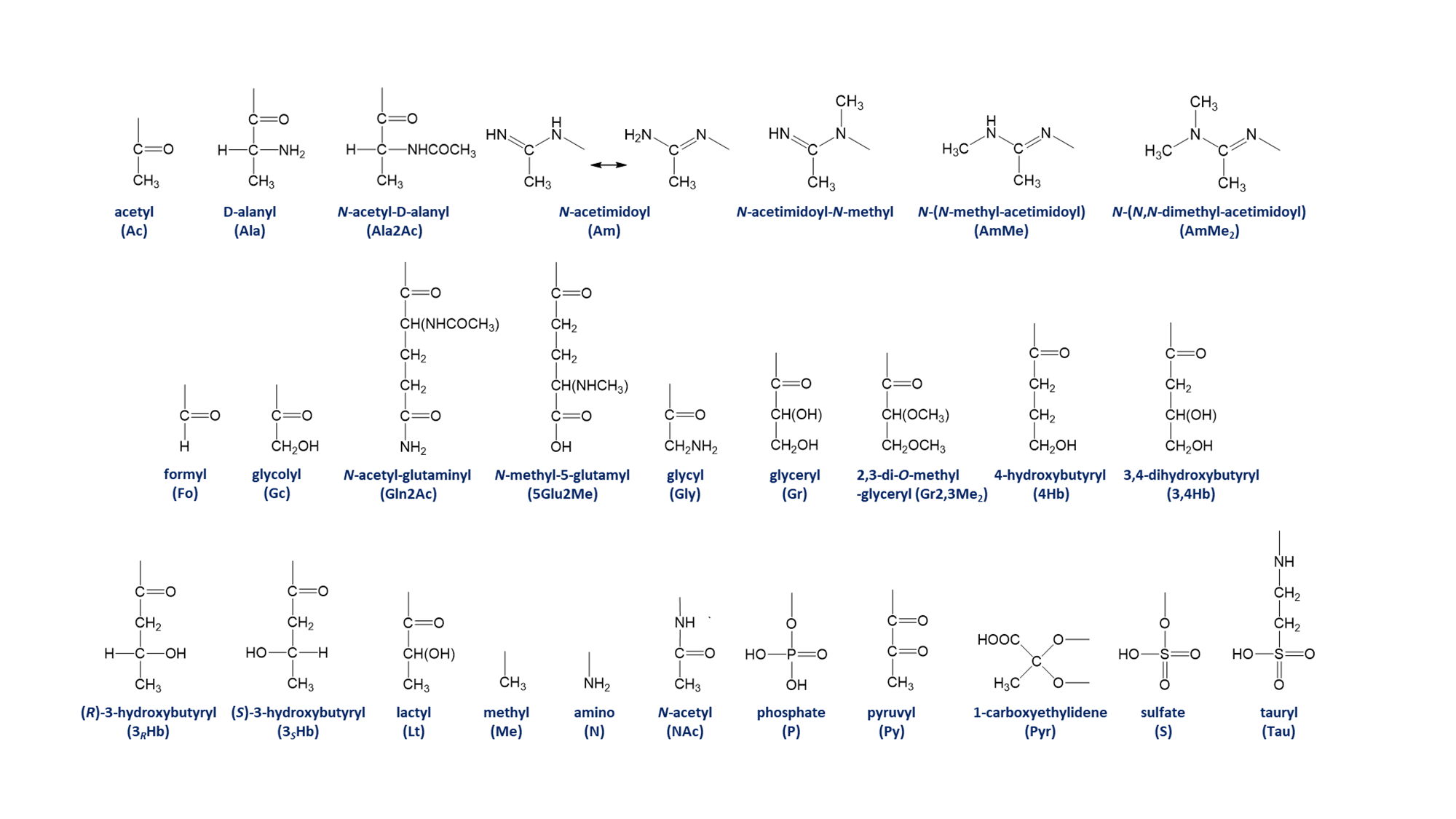
Table 3. Monosaccharide substituents and abbreviations* ☝
| Abbreviation | Substituent |
|---|---|
| Ac | acetyl |
| Ala | D-alanyl |
| Ala2Ac | N-acetyl-D-alanyl |
| Am | N-acetimidoyl |
| AmMe | N-(N-methyl-acetimidoyl) |
| AmMe2 | N-(N,N-dimethyl-acetimidoyl) |
| Fo | formyl |
| Gc | glycolyl |
| Gln2Ac | N-acetyl-glutaminyl |
| 5Glu2Me | N-methyl-5-glutamyl |
| Gly | glycyl |
| Gr | glyceryl |
| Gr2,3Me2 | 2,3-di-O-methyl-glyceryl |
| 4Hb | 4-hydroxybutyryl |
| 3,4Hb | 3,4-dihydroxybutyryl |
| 3RHb | (R)-3-hydroxybutyryl |
| 3SHb | (S)-3-hydroxybutyryl |
| Lt | lactyl |
| Me | methyl |
| N | amino |
| NAc | N-acetyl |
| P | phosphate |
| Py | pyruvyl |
| Pyr | 1-carboxyethylidene |
| S | sulfate |
| Tau | tauryl |
*Substituents listed with saccharides are listed in alphabetical order based on common usage and IUPAC nomenclature (Carbohydr. Res., 1997, 297, 1-90; https://www.qmul.ac.uk/sbcs/iupac/2carb/)
Chemical structure of substituents ☝
Table 4. Monosaccharide abbreviations and names ☝
| Abbreviation | Short Name | Systematic name |
|---|---|---|
| 4eLeg | 4-epi-Legionaminic acid | 5,7-Diamino-3,5,7,9-tetradeoxy-D-glycero-D-talo-non-2-ulopyranosonic acid |
| 6dAlt | 6-Deoxy-L-altrose | 6-Deoxy-L-altropyranose |
| 6dAltNAc | N-Acetyl-6-deoxy-L-altrosamine | 2-Acetamido-2,6-dideoxy-L-altropyranose |
| 6dGul | 6-Deoxy-D-gulose | 6-Deoxy-D-gulopyranose |
| 6dTal | 6-Deoxy-D-talose | 6-Deoxy-D-talopyranose |
| 6dTalNAc | N-Acetyl-6-deoxy-D-talosamine | 2-Acetamido-2,6-dideoxy-D-talopyranose |
| 8eAci | 8-epi-Acinetaminic acid | 5,7-Diamino-3,5,7,9-tetradeoxy-D-glycero-L-altro-non-2-ulopyranosonic acid |
| 8eLeg | 8-epi-Legionaminic acid | 5,7-Diamino-3,5,7,9-tetradeoxy-L-glycero-D-galacto-non-2-ulopyranosonic acid |
| Abe | Abequose | 3,6-Dideoxy-D-xylo-hexopyranose |
| Aci | Acinetaminic acid | 5,7-Diamino-3,5,7,9-tetradeoxy-L-glycero-L-altro-non-2-ulopyranosonic acid |
| All | D-Allose | D-Allopyranose |
| AllA | D-Alluronic acid | D-Allopyranuronic acid |
| AllN | D-Allosamine | 2-Amino-2-deoxy-D-allopyranose |
| AllNAc | N-Acetyl-D-allosamine | 2-Acetamido-2-deoxy-D-allopyranose |
| Alt | L-Altrose | L-Altropyranose |
| AltA | L-Altruronic acid | L-Altropyranuronic acid |
| AltN | L-Altrosamine | 2-Amino-2-deoxy-L-altropyranose |
| AltNAc | N-Acetyl-L-altrosamine | 2-Acetamido-2-deoxy-L-altropyranose |
| Api | L-Apiose | 3-C-(Hydroxymethyl)-L-erythro-tetrofuranose |
| Ara | L-Arabinose | L-Arabinopyranose |
| Bac | Bacillosamine | 2,4-Diamino-2,4,6-trideoxy-D-glucopyranose |
| Col | Colitose | 3,6-Dideoxy-L-xylo-hexopyranose |
| DDmanHep | D-glycero-D-manno-Heptose | D-glycero-D-manno-Heptopyranose |
| Dha | 3-Deoxy-D-lyxo-heptulosaric acid | 3-Deoxy-D-lyxo-hept-2-ulopyranosaric acid |
| Dig | D-Digitoxose | 2,6-Dideoxy-D-ribo-hexopyranose |
| Fru | D-Fructose | D-arabino-Hex-2-ulopyranose |
| Fuc | L-Fucose | 6-Deoxy-L-galactopyranose |
| FucNAc | N-Acetyl-L-fucosamine | 2-Acetamido-2,6-dideoxy-L-galactopyranose |
| Gal | D-Galactose | D-Galactopyranose |
| GalA | D-Galacturonic acid | D-Galactopyranuronic acid |
| GalN | D-Galactosamine | 2-Amino-2-deoxy-D-galactopyranose |
| GalNAc | N-Acetyl-D-galactosamine | 2-Acetamido-2-deoxy-D-galactopyranose |
| Glc | D-Glucose | D-Glucopyranose |
| GlcA | D-Glucuronic acid | D-Glucopyranuronic acid |
| GlcN | D-Glucosamine | 2-Amino-2-deoxy-D-glucopyranose |
| GlcNAc | N-Acetyl-D-glucosamine | 2-Acetamido-2-deoxy-D-glucopyranose |
| Gul | D-Gulose | D-Gulopyranose |
| GulA | D-Guluronic acid | D-Gulopyranuronic acid |
| GulN | D-Gulosamine | 2-Amino-2-deoxy-D-gulopyranose |
| GulNAc | N-Acetyl-D-gulosamine | 2-Acetamido-2-deoxy-D-gulopyranose |
| Ido | L-Idose | L-Idopyranose |
| IdoA | L-Iduronic acid | L-Idopyranuronic acid |
| IdoN | L-Idosamine | 2-Amino-2-deoxy-L-idopyranose |
| IdoNAc | N-Acetyl-L-idosamine | 2-Acetamido-2-deoxy-L-idopyranose |
| Kdn | 2-Keto-3-deoxy-nononic acid | 3-Deoxy-D-glycero-D-galacto-non-2-ulopyranosonic acid |
| Kdo | 3-Deoxy-D-manno-octulosonic acid | 3-Deoxy-D-manno-oct-2-ulopyranosonic acid |
| Leg | Legionaminic acid | 5,7-Diamino-3,5,7,9-tetradeoxy-D-glycero-D-galacto-non-2-ulopyranosonic acid |
| LDmanHep | L-glycero-D-manno-Heptose | L-glycero-D-manno-Heptopyranose |
| Lyx | D-Lyxose | D-Lyxopyranose |
| Man | D-Mannose | D-Mannopyranose |
| ManA | D-Mannuronic acid | D-Mannopyranuronic acid |
| ManN | D-Mannosamine | 2-Amino-2-deoxy-D-mannopyranose |
| ManNAc | N-Acetyl-D-mannosamine | 2-Acetamido-2-deoxy-D-mannopyranose |
| Mur | Muramic acid | 2-Amino-3-O-[(R)-1-carboxyethyl]-2-deoxy-D-glucopyranose |
| MurNAc | N-Acetylmuramic acid | 2-Acetamido-3-O-[(R)-1-carboxyethyl]-2-deoxy-D-glucopyranose |
| MurNGc | N-Glycolylmuramic acid | 3-O-[(R)-1-Carboxyethyl]-2-deoxy-2-hydroxyacetamido-D-glucopyranose |
| Neu | Neuraminic acid | 5-Amino-3,5-dideoxy-D-glycero-D-galacto-non-2-ulopyranosonic acid |
| Neu5Ac | N-Acetylneuraminic acid | 5-Acetamido-3,5-dideoxy-D-glycero-D-galacto-non-2-ulopyranosonic acid |
| Neu5Gc | N-Glycolylneuraminic acid | 3,5-Dideoxy-5-hydroxyacetamido-D-glycero-D-galacto-non-2-ulopyranosonic acid |
| Oli | Olivose | 2,6-Dideoxy-D-arabino-hexopyranose |
| Par | Paratose | 3,6-Dideoxy-D-ribo-hexopyranose |
| Pse | Pseudaminic acid | 5,7-Diamino-3,5,7,9-tetradeoxy-L-glycero-L-manno-non-2-ulopyranosonic acid |
| Psi | D-Psicose | D-ribo-Hex-2-ulopyranose |
| Qui | D-Quinovose | 6-Deoxy-D-glucopyranose |
| QuiNAc | N-Acetyl-D-quinovosamine | 2-Acetamido-2,6-dideoxy-D-glucopyranose |
| Rha | L-Rhamnose | 6-Deoxy-L-mannopyranose |
| RhaNAc | N-Acetyl-L-rhamnosamine | 2-Acetamido-2,6-dideoxy-L-mannopyranose |
| Rib | D-Ribose | D-Ribopyranose |
| Sia | Sialic acid | Sialic acid residue of unspecified type |
| Sor | L-Sorbose | L-xylo-Hex-2-ulopyranose |
| Tag | D-Tagatose | D-lyxo-Hex-2-ulopyranose |
| Tal | D-Talose | D-Talopyranose |
| TalA | D-Taluronic acid | D-Talopyranuronic acid |
| TalN | D-Talosamine | 2-Amino-2-deoxy-D-talopyranose |
| TalNAc | N-Acetyl-D-talosamine | 2-Acetamido-2-deoxy-D-talopyranose |
| Tyv | Tyvelose | 3,6-Dideoxy-D-arabino-hexopyranose |
| Xyl | D-Xylose | D-Xylopyranose |
Clicking on the abbreviation leads to the corresponding entry in PubChem
Updates ☝
June 27, 2022 (Version 2.0)
- SNFG expanded to describe sialic acid and sialic acid-like monosaccharides.
- Substituent table added, along with figure describing their chemical structures.
- SNFG notes expanded to include new guidelines on how to describe substituents.
- SNFG examples and presentation slides redone with more examples, and refinements.
- SNFG Table 1 redone with higher resolution images.
- SNFG versioning initiated.
- Rules added to describe open ring configurations (aldehyde, ketone) at free reducing end.
February 4, 2020 (Version 1.5)
- Rules added for specification of symbol and bond orientation.
November 20, 2019 (Version 1.4)
- SNFG now provides guidelines to depict ambiguous linkages and glycan mixtures using bracket notation.
May 9, 2019 (Version 1.3)
- Footnote 7 modified to accommodate variable modifications.
- More SNFG usage examples added.
January 22, 2019 (Version 1.2)
- SNFG page reorganized with new examples from mammals, yeast, slime mold, insects, bacteria and plants.
- The new examples focus on how to present carbohydrate structures in diverse organisms and ambiguous monosaccharide assignments.
- Number of footnotes reduced from 28 to 10, and thematically organized in order to simplify usage.
- Single non-italicized letter is now allowed in white symbols to help describe monosaccharides that are not part of the SNFG table and that cannot be described using existing footnotes.
- SNFG Discussion Group list updated.
June 5, 2017 (Version 1.1)
- SNFG Discussion Group Listing added.
- White diamond now indicates any deoxynonulosonic acid.
- Flattened diamond introduced for any dideoxynonulosonic acid.
- Additional symbols added for 6dGul, 6dAltNAc, 6dTalNAc, Pse, Leg, Aci and 4eLeg.
- Drawglycan-SNFG and Glycanbuilder 2-SNFG adopted, link to web site provided.
- Updates to Appendix 52A. Organizations and publications adopting SNFG.
- RGB color code provided in addition to CMYK
- Multiple minor corrections and additions to nomenclature and rules of display.
August 31, 2016 (Version 1.0)
- Link to new Appendix 52A. Organizations and Publications Adopting SNFG.
- Red diamond introduced for Sia (sialic acid, type unspecified). White diamond indicates any nonulosonic acid.
- A 3D Symbol Nomenclature for Glycans (3D-SNFG) adopted, link to web site provided.
- Multiple minor corrections and additions to nomenclature and rules of display.