NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Anderson M, Panteli D, Mossialos E. How can the EU support sustainable innovation and access to effective antibiotics? Policy options for existing and new medicines [Internet]. Copenhagen (Denmark): European Observatory on Health Systems and Policies; 2023. (Policy Brief, No. 51.)

How can the EU support sustainable innovation and access to effective antibiotics? Policy options for existing and new medicines [Internet].
Show details1. Introduction
Growing antibiotic resistance poses a major threat to public health
Antibiotics, and other antimicrobials such as antifungals and antivirals, are one of the greatest achievements of modern medicine.1 Antibiotics have not just revolutionized the treatment of bacterial infections, but also facilitated breakthroughs in other areas of healthcare, such as surgery and oncology. However, growing antibiotic resistance means these breakthroughs are being jeopardized and urgent action is needed to tackle this growing public health threat. The scope of this policy brief is predominantly to focus on antibiotics and antibiotic resistance. However, many of the issues and policy responses discussed in this brief overlap with the broader terms antimicrobials and antimicrobial resistance (AMR).
The health impact of antibiotic resistance is similar to that of influenza, tuberculosis and HIV/AIDS combined
The health and economic impacts of antibiotic resistance are a major challenge for all healthcare systems and societies. Different estimates exist, but there is agreement that antibiotic-resistant infections are responsible for a substantial number of deaths in Europe each year. A review of surveillance data from 2016–2020 collated by the European Centre for Disease Prevention and Control (ECDC) found that each year in the EU/EEA resistant infections result in more than 35,000 deaths and more than 1 million years of life lost because of premature death or years lived with disabilities (ECDC, 2022a). This health burden is comparable to that of influenza, tuberculosis and HIV/AIDS combined (Cassini et al., 2018). Antibiotic-resistant bacterial infections impact all Member States, although the health burden is higher in Member States from southern and eastern Europe (Figure 1).
Globally, antibiotic resistance is a leading cause of mortality, but its health burden is unevenly distributed
Taking a global perspective, the health burden of antibiotic resistance is highest in Western Sub-Saharan Africa and South Asia, with a death rate attributed to antibiotic resistance of 27.3 per 100,000 and 21.5 per 100,000 respectively in 2019 (Figure 2) (Murray et al., 2022). This is significantly higher than in Western Europe, where the death rate attributed to antibiotic resistance was 11.7 per 100,000, although comparable to Eastern Europe, which had a death rate of 19.9 per 100,000. In 2019, it has been estimated that antibiotic resistance was directly responsible for 1.27 million deaths (equivalent to approximately 3,500 people every day). This means that drug-resistant bacterial infections were also responsible for more deaths than both HIV/AIDS (864,000 deaths) or malaria (643,000 deaths) (Murray et al., 2022).
Antibiotic resistance also results in high healthcare costs and productivity losses
From an economic perspective, it is estimated that antibiotic resistance costs about €1.1 billion to the healthcare systems of EU/EEA countries (OECD, 2019). The treatment cost of a resistant infection has been estimated to be between US$10,000 and US$40,000 higher than that of a susceptible infection (Cohen et al., 2010; Smith & Coast, 2013; Tansarli et al., 2013). The additional healthcare costs are driven by a variety of complex and interacting factors. First, the use of second-line antibiotics, or different combinations of antibiotics before the most effective strategy is identified, can increase pharmaceutical expenditure. Second, the higher use of diagnostics and laboratory tests to identify effective treatments and monitor complications is associated with increased costs. Third, patients with antibiotic-resistant infections are more likely to require hospitalization and critical care support. Fourth, antibiotic-resistant bacterial infections may increase the probability of requiring surgery, for example the removal of infected tissue or amputation. Lastly, antibiotic-resistant bacterial infections are associated with substantial increases in length of hospital stay. The Organisation for Economic Co-operation and Development (OECD) has modelled the economic impact of AMR in the future and estimates that the cumulative costs of AMR to the healthcare systems of EU and OECD countries are expected to reach US$134 billion by 2050 (OECD, 2018).
Broad policies across human, animal and environmental health are required to curb growth of antibiotic resistance
As bacteria are exposed to antibiotics in human, animal and environmental health settings, and may spread between these, the policy responses to antibiotic resistance should take a ‘One Health’ perspective (Forman et al., 2022). This means targeting actions towards the public as well as the healthcare professionals, researchers, veterinarians, farmers and actors involved in different parts of waste management (Table 1). Improving awareness of antibiotic resistance through both public awareness campaigns and education of relevant professional groups is key to reducing inappropriate use of antibiotics. Strengthening surveillance is key in order to monitor the usage of antibiotics as well as track trends in the prevalence and incidence of resistant bacterial infections. Promoting antibiotic stewardship includes ensuring that the right antibiotic is used for the right infection at the right time. Infection prevention and control (IPC) is a combination of interventions such as improved sanitation, hand hygiene and vaccination, which aim to prevent the emergence and transmission of healthcare-associated infections (HAI) and resistant bacteria. Finally, fostering R&D of new antibiotics is important in order to be able to replace those that have been rendered ineffective by resistant infection. In addition, the R&D of new diagnostics and vaccines can significantly reduce the unnecessary use of antibiotics and prevent infections.
Table 1
Tackling antibiotic resistance requires targeting actions towards a broad range of stakeholders.
This policy brief looks specifically at how actions at the EU level can be strengthened to support sustainable access to effective pre-existing and novel antibiotics
While developing holistic and multicomponent strategies in accordance with the ‘One Health’ perspective is key to curbing the development and spread of antibiotic resistance, this policy brief is specifically focused on antibiotic R&D for human health and how actions at the EU level can be strengthened to secure sustainable access to pre-existing and new antibiotics. The brief considers this challenge across the drug development pathway, from stimulating R&D, to registration of antibiotics, and finally the manufacture and supply of antibiotics. The policy brief is structured in accordance with the following policy questions:
- What are the major challenges across the R&D pathway for antibiotics?
- What challenges exist to ensure the sustainable supply of new and pre-existing antibiotics?
- What actions can be taken at the EU level to stimulate research, innovation and development of new antibiotics?
- How can the EU promote sustainable and timely access to pre-existing and new antibiotics once they have been developed and approved?
Box 1
How was the evidence that is summarized in this brief compiled?
2. What are the major challenges across the R&D pathway for antibiotics?
This chapter reviews the antibiotic pipeline by first looking at newly approved antibiotics, and then antibiotics currently under development. We then discuss the barriers to stimulating innovation, research and development into novel antibiotics.
2.1. Newly approved antibiotics
Few newly approved antibiotics are innovative, which makes them susceptible to cross-resistance with pre-existing antibiotics
Following a particularly active period of antibiotic R&D from 1940 to 1980, there have been relatively few new antibiotics developed over the last three decades (Renwick & Mossialos, 2018). The new antibiotics that have received market approval in recent years have often been adaptations of pre-existing antibiotics and therefore vulnerable to cross-resistance (Mossialos et al., 2010b). This is important as the more unique a new antibiotic is compared to existing antibiotic structures (Box 2), the likelier it is that resistance development will take longer, as pathogens are unlikely to have previously encountered the chemical components of the antibiotic (WHO, 2017b).
Box 2
The WHO evaluates new antibiotics according to innovation and priority criteria.
Between 2017 and 2021, 12 new antibiotics were approved (Table 3). Generally, these new antibiotics do not meet the aforementioned innovation criteria. There is inconclusive data regarding whether they show evidence of cross-resistance to existing antibiotic classes, and only two of the antibiotics incorporated characteristics which might warrant a new chemical classification. None of these antibiotics had a new target or mechanism of action. Only one new antibiotic is effective against CRAB and CRPA pathogens. Five new antibacterials work against CRE pathogens, and seven new antimicrobials work against other priority pathogens. The WHO 2021 overview and analysis of the antibiotic pipeline describes the supply of recently approved antibiotics as “insufficient” to address the growing threat of antibiotic resistance (WHO, 2022a).
Table 3
Only one new antibiotic from those that gained market authorization between 2017 and 2021 is effective against critical priority CRAB and CRPA pathogens and only two are somewhat innovative.
2.2. Antibiotics under development
There were 77 antibiotics under development in 2021, but few of them are innovative
The current antibiotic pipeline as of 2021 includes 45 traditional antibacterial agents and 32 non-traditional antibacterial agents such as antibodies, bacteriophages and immunomodulation agents. Similar to recently approved antibiotics, most antibiotics in the clinical phase of development do not demonstrate innovative characteristics. Although 27 of the 45 traditional antibiotics target WHO priority pathogens, only six meet at least one of the four WHO innovation criteria. Only two target at least one of the pathogens considered to be a ‘critical’ priority. The remaining traditional antibiotics target either tuberculosis (TB) (13/45) or Clostridioides difficile (5/45). There appear to be more innovative characteristics in antibiotics under development that target these pathogens. Six antibiotics under development to target TB demonstrate “absence of cross-resistance with existing antibiotics” and seven represent a new chemical class. Four of the five antibiotics under development to target C. difficile demonstrate at least one innovative criterion, with two meeting all innovative criteria. Approximately half of these agents were in the first phase of clinical development (44%; 34/77), a third were in the second phase (38%; 29/77), and a sixth were in the third phase (18%; 14/77) (Figure 3).

Figure 3
Traditional and non-traditional antibacterial agents by clinical development phase. Source: WHO, 2022a.
In contrast to the clinical pipeline, and in large parts thanks to the use of push incentives over the last decade, the preclinical pipeline has been described as “innovative and including a large number of non-traditional approaches” (WHO, 2022a). Moreover, the WHO has noted that “preclinical pipeline projects are broadly distributed geographically, and there is a large variety of product types” (see Tables 8 and 9 of the WHO 2021 antibiotic pipeline analysis; WHO, 2022a). Yet, as we will describe in the next section, preclinical product developers experience difficulties in securing funding for early product development, when the risk of failure is particularly high compared to advanced clinical studies. This is why their vulnerabilities and turnover rates are very high.
2.3. Barriers to stimulating innovation, research and development into novel antibiotics
Many drugs at the early stages of clinical development never obtain market approval
While it may seem that there are many antibiotics under development, it is important to note that many drugs at the early stages of clinical development never obtain market approval. A review of literature on transition probabilities for antibiotics in development found that success rates for antibiotics between phase 1 and 2 was 33.3%, between phase 2 and 3 was 75.0%, between phase 3 and submission for market approval was 85.7%, and between submission and obtaining market approval was 75.0% (Figure 4).
Collectively, these transition probabilities mean that only around one in six antibiotics that enter phase 1 trials subsequently obtain market approval (Figure 4). However, the success rate of moving an antibiotic through preclinical research to market approval is much lower. The success rate across the combined preclinical and clinical pathway has been estimated to be as low as between 1.5–3.5% and can take up to 15 years (Review on Antimicrobial Resistance, 2016). This is caused by a complex range of economic, regulatory, structural and scientific factors that impact many stages of the drug development pathway (Renwick, Brogan & Mossialos, 2016a; Theuretzbacher et al., 2022).
Most antibiotic R&D is undertaken by SMEs with limited financing
Preclinical research aims to identify new molecules that have potential to function as antibiotic agents. Several scientific challenges exist in developing new chemical classes or mechanisms of action against resistant bacteria. Despite the sequencing of the first complete bacterial genome, completed as early as 1995, scientists have been largely unsuccessful in screening target genes to identify drug candidates for new antibiotics (Payne et al., 2007). Instead, developing novel chemical structures that inhibit validated cellular targets appears to be a more promising strategy for developing compounds capable of destroying resistant bacteria. However, discovering novel chemical structures that are safe for human consumption is technically challenging. Moreover, destroying Gram-negative rather than Gram-positive bacteria has proven particularly challenging as Gram-negative bacteria have a protective outer membrane (Miller, 2016). A lack of scientific and technical expertise in antibiotic research also limits progress in this area (Silver, 2011; Theuretzbacher et al., 2022). From a funding perspective, much of the basic science and discovery research is undertaken by academic labs and SMEs that have limited funding available, meaning that many preclinical projects are delayed or abandoned due to financial issues. In 2021, the WHO identified 217 preclinical projects focused on antibiotic development, involving 121 institutions (WHO, 2022a). Of these 121 institutions, 85.1% (103) were companies, 13.2% (16) were academic institutions, and 1.7% (2) were foundations; 49.5% (51) of these companies had fewer than 10 employees (Figure 5).

Figure 5
Most preclinical antibiotic research is undertaken by SMEs. Note: This figure shows the categorization of companies with preclinical pipeline projects by ownership and size. Source: WHO, 2022a.
Once promising antibiotic candidates have been identified, they can move to clinical trial phases if financial support is available. However, these clinical trials can be logistically challenging to conduct due to short treatment windows and lack of rapid point-of-care diagnostics to identify potential participants. Similar to the preclinical research, the majority of clinical development of antibiotic candidates is conducted by SMEs. A survey conducted in 2021 indicated that 75% (41/55) of projects active in late-stage (phase 2 or later) antibiotic development had been developed by SMEs (Access to Medicine Foundation, 2021). As SMEs typically have limited financial and human resources, as well as technical expertise, they sometimes struggle to coordinate or set up clinical trials. In some cases, a SME cannot take the financial risk of moving an antibiotic candidate through to the clinical trial phase, or the development of an antibiotic candidate is significantly delayed while the SME seeks external funding support. There is also a particularly high turnover of SMEs involved in antibiotic development, with many SMEs becoming bankrupt or being dissolved each year. For example, 33.8% (46/136) of developers involved in antibiotic development that responded to a 2020 survey did not respond in the following year, and are now presumed to be inactive (WHO, 2022a).
Investing in antibiotic R&D is financially risky, with a lower expected return on investment than other therapeutic areas
Launching and commercializing a new antibiotic is a financially risky prospect and many antibiotic developers have subsequently experienced significant financial losses or filed for bankruptcy (ReAct, 2021). The expected return on investment of a drug development project can be expressed by the expected net present value (NPV), which is a sum of all expected revenues and costs of a project adjusted for the value of money over time and risk of failure. The expected NPV of an antibiotic development project has been estimated to be US$ –50 million, in comparison to US$ +720 million for a neurological drug and US$ +1.15 billion for a musculoskeletal drug (Sharma and Towse, 2011). An analysis of sales data from developed countries also showed that spending on antibiotics has declined between 2011 and 2020, making the antibiotic market even less attractive for the pharmaceutical industry (Figure 6).
Greater transparency about R&D costs could help design appropriately sized incentives
Estimates of the financial cost of developing a new antibiotic vary considerably. The Review on Antimicrobial Resistance estimated the collective cost of phase 1, 2 and 3 clinical trials in 2015 to be approximately US$133 million, and the cost of post-approval trials to be approximately US$146 million (Review on Antimicrobial Resistance, 2016). In comparison, in 2020, the Wellcome Trust estimated the total cost of antibiotic R&D, including preclinical research, clinical trials and market launch, to be as high as US$1.8 billion for an existing-class antibiotic, and US$2.6 billion for a new-class antibiotic (Wellcome Trust, 2020). Outterson conducted a review of cost studies and estimated that, in 2021, the total cost of R&D for a new antibiotic, including preclinical research, clinical trials and post-approval was US$447 million (Outterson, 2021). Moving forward, greater transparency from antibiotic developers regarding the costs of R&D would assist in designing appropriately sized incentives, particularly as the R&D costs for different antibiotics vary significantly and are influenced by factors such as target pathogens and clinical indications.
Overcoming the technical and regulatory burdens is a major challenge during the marketing approval stage
Once an antibiotic candidate reaches the stage of applying for marketing approval, there are several other hurdles to overcome. There are procedural differences between national and regional drug regulatory agencies in approving novel antibiotics, including differences in patient selection criteria, specification of statistical parameters, definitions of clinical endpoints, and rules regarding expedited approvals, which makes global licensing both time-consuming and costly (Stern et al., 2017). Companies launching new antibiotics also need the technical competences to submit applications to all the relevant regulatory agencies in a timely manner that meets the necessary requirements – and this is again particularly challenging for SMEs, which typically have limited human resources. In contrast, in other therapeutic areas, there is greater involvement of large pharmaceutical companies that have the technical and regulatory expertise to guide new medicines to regulatory approval.
Conventional HTAs do not consider many dimensions of value relevant to antibiotics
Once regulatory approval has been granted, in some countries, companies are then required to undergo HTA processes to demonstrate the value of newly licensed antibiotics in relation to pre-existing treatments. However, conventional HTA processes do not consider many additional elements of value specific to antibiotics, such as the benefits of avoiding the spread of infection, and enabling treatments in other therapeutic areas (Box 3). This may contribute to a lack of confidence for pharmaceutical companies as to whether they will receive adequate return on investment following investment in the R&D of new antibiotics (Colson et al., 2021).
Box 3
Additional value elements specific to antibiotics have been proposed for inclusion in HTA considerations.
3. What challenges exist to ensure sustainable supply of new and pre-existing antibiotics?
This chapter is concerned with the challenges that exist around ensuring a sustainable supply of new and pre-existing antibiotics. First, we consider how limited access to pre-existing and new antibiotics can harm patients and drive antibiotic resistance. Second, we explore the driving factors behind limited access to newly approved antibiotics. Third, we look at the driving factors behind limited access and shortages of pre-existing generic antibiotics.
3.1. How does limited access to pre-existing and new antibiotics harm patients and drive antibiotic resistance?
Limited access to antibiotics can lead to the use of suboptimal antibiotics or substitution of narrow-spectrum for broad-spectrum – and both drive resistance
Due to economic factors (e.g., manufacturers often find it is not commercially viable to supply certain antibiotics to some populations), or to shortages, many countries have limited access to both pre-existing and new antibiotics. Failing access to appropriate and effective antibiotics puts patients at risk and may lead to increased morbidity and mortality. Further, this can impact resistance development in several ways (ReAct, 2020b). First, this means that suboptimal antibiotics that may not be as effective are used to treat infections (Miljković et al., 2022), resulting in prolonged infections and more opportunities for resistant bacterial infections to spread. Second, particularly harmful mutations in genetic structure can occur if narrow-spectrum antibiotics (intended to be used for specific indications) have to be substituted for broad-spectrum antibiotics (intended to be used to treat a wide range of infections). This is because broad-spectrum antibiotics exert a wider ‘selection pressure’ and their use encourages strains of bacteria that are resistant to multiple types of antibiotics to develop. To guide policy, the WHO has developed the Access, Watch and Reserve (AWaRe) classification to classify antibiotics according to risk of resistance development (Box 4) (WHO, 2021a).
Box 4
WHO AWaRe classification of antibiotics differentiates between three levels of risk for resistance development.
The number of countries using at least 60% of antibiotics with low risk for resistance development has decreased in Europe
It is known that the use of ‘Access’ antibiotics exerts lower ‘selection pressure’2 than ‘Watch’ antibiotics (Sulis et al., 2022), and the WHO has defined a target where at least 60% of human antibiotic used at the country level should be ‘Access’ antibiotics (WHO, 2019). Unfortunately, the number of countries meeting this target has decreased over the last two decades (Klein et al., 2021). In 2020, eight Member States did not meet this target (Bulgaria, 41%; Cyprus, 44%; Slovakia, 44%; Italy, 47%; Greece, 49%; Romania, 50%; Hungary, 51%; Malta, 55%) (ECDC, 2022b). Third, shortages of antibiotics create a market opportunity for substandard or falsified antibiotics (Cecchini & Lee, 2017). These poor-quality antibiotics can result in prolonged infections and more potential for resistance to emerge. The WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products has identified several instances of substandard or falsified antibiotics in Europe and internationally (WHO, 2017a). However, more research is needed to quantitatively assess the impact of unavailability of pre-existing and new antibiotics on resistance development through these different mechanisms.
3.2. What challenges exist to providing sustainable supply of new antibiotics?
Return on investment for manufacturing and commercializing new antibiotics is much lower compared to other therapeutic areas
Once market approval has been granted, companies are expected to continue to manufacture and commercialize new antibiotics. However, the return on investment for doing so is smaller relative to other therapeutic areas such as neurologic, diabetes and cardiovascular drugs (Review on Antimicrobial Resistance, 2016). Despite their significant value for broader public health, the intrinsic nature of antibiotic treatment means they are unlikely to generate significant revenue. This is because antibiotics typically have short durations of treatment, and they are subject to stewardship efforts to minimize usage to reduce further development and spread of resistance. New antibiotics typically receive regulatory approval based on data from non-inferiority clinical trials, which means that physicians are unsure whether new antibiotics are an improvement compared to pre-existing treatments. As a result, new antibiotics are not immediately featured within clinical guidance. Moreover, the significant crossover in application between novel antibiotics and pre-existing antibiotics (which are typically off-patent/generics) places downward pressure on prices.
Many new antibiotics are only launched in larger markets
In practice, many antibiotic developers choose only to launch new antibiotics in larger markets as the costs to launch in smaller markets are often too high compared to the revenues achievable in those markets. An analysis of 18 new antibiotics launched in G7 and seven other high-income countries between 2010 and 2019 found that the majority of newly approved antibiotics were only available in the US, UK and Sweden (Outterson et al., 2022b).3 They also found that the EMA approval did not lead to an automatic launch in Europe, and that the median annual sales for antibiotics in the first year after launch was only US$16.2 million. Another study, commissioned by Merck & Co, which focused on reimbursement of five new antibiotics that had received regulatory approval by the EMA since 2015, found that all five antibiotics were only available in five Member States, and that the average time from market authorization to reimbursement varied from 165 days in Austria to 1401 days in Belgium (Figure 7). Collectively, these market failures have resulted in several antibiotic companies that have successfully brought new antibiotics to market either filing for bankruptcy or ceasing operations (ReAct, 2021).
3.3. What challenges exist to providing sustainable supply of pre-existing generic antibiotics?
Shortages of essential antibiotics are a major issue in many countries
While many challenges for suppliers exist during the market launch and commercialization of new antibiotics, there are also challenges with unpredictable supply and shortages of pre-existing and older antibiotics. Antibiotic shortages are a major issue in all countries. In 2019, there were over 1,300 notifications of antibiotic shortages across the EU (although definitions of shortages and notification requirements varied between Member States) (European Commission et al., 2021). Shortages of ‘Access’ antibiotics, such as amoxicillin and clindamycin, are also commonly reported by Member States (European Commission et al., 2021). Systemic anti-infectives were the third most frequent class of medicines for which shortages were reported (Figure 8).
Shortages of pre-existing antibiotics can have long-term negative impacts on stewardship efforts
In 2020, the EU Joint Action on Antimicrobial Resistance and Healthcare-Associated Infections (EU-JAMRAI) conducted interviews with 13 high-income countries in Europe, Canada, South Africa and Japan to understand to what extent shortages of antibiotics are an issue (EU-JAMRAI, 2021). Out of the 13 countries, 12 indicated that shortages of pre-existing antibiotics are a serious problem in their country, and eight out of 13 indicated that this resulted in greater use of broad-spectrum antibiotics as substitutes. Interestingly, several countries highlighted the long-term negative impact of antibiotic shortages on stewardship efforts as physicians often change their prescribing habits and reduce compliance with evidence-informed prescribing guidelines. One of the most prominent and severe examples of an antibiotic shortage, which demonstrated the insecurity of supply of antibiotics, has been the international and prolonged shortage of piperacillin-tazobactam experienced following an explosion in a Chinese factory that was responsible for producing most of the world’s supply of the API necessary for its production. This has frequently resulted in substitution with antibiotics with a broader spectrum of activity, such as cephalosporins, thereby promoting resistance development and placing patients at higher risk of hospital-associated Clostridium difficile infections (Gross et al., 2017).
Shortages often occur due to the limited number of actors involved in the production and supply chain
Antibiotic shortages typically occur because there are limited actors involved in the production and supply chain, and it has not been possible to forecast and anticipate shortages and demand for the antibiotics (Shafiq et al., 2021). The antibiotic manufacturing supply chain involves several steps, including generating raw materials, developing reaction intermediates, producing API, converting to medicinal products and packaging (Figure 9).

Figure 9
The antibiotic manufacturing supply chain involves several stages. Source: Adapted from BCG & Wellcome Trust, 2021.
The production of APIs and converting them to medicinal products are the most vulnerable stages in the antibiotic supply chain
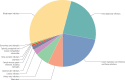
The most vulnerable stages of antibiotic production are API production and converting those APIs to medicinal products. This is because most API production takes place in India and China, and in large quantities to achieve economies of scale and maximize profits. A global analysis of 40 antibiotics found that close to 70% of the manufacturing sites for antibiotic APIs are located in India (35%) and China (34%) (BCG & Wellcome Trust, 2021). China is also the largest exporter of antibiotic APIs, accounting for 71% of international exports in 2020 (Figure 10).
Figure 10
China is the largest exporter of antibiotic APIs globally. Note: This figure shows globally exported API volumes for antibiotics, in kilotonnes, for 2020. Source: BCG & Wellcome Trust, 2021.
Supply of many essential antibiotics is dependent on a few key API suppliers in China, with the number of manufacturers converting APIs to medicinal products also limited
This means that many generic antibiotics are produced using a single-source API, and the global supply of many essential antibiotics, such as cephalosporins, macrolides and penicillins, are dependent upon a few key API suppliers in China (TIFAC, 2020). Therefore, any disruption to supply caused by accidents or facilities shutting down, or trade restrictions, can cause major global shortages (as demonstrated by the international shortages of piperacillin-tazobactam). There are also very few generic manufacturers that convert API to medicinal products because purchasers often procure generic antibiotics from the supplier that offers the lowest price and, over time, it is not economically feasible for smaller generic manufacturers to stay in the market.
Monitoring and forecasting the antibiotic supply chain and demand is insufficient and complicated by a lack of transparency about its configuration
There is also a vacuum in responsibility at the international and national levels in relation to monitoring and forecasting the antibiotic supply chain and demand. This is further complicated by lack of transparency about the configuration of supply chains, starting from the actual manufacturers beyond market authorization holders and stretching to the relationship of manufacturers with API suppliers, as this information is typically understood to be commercially sensitive. However, some countries, such as New Zealand, have taken steps to address this by publishing the sites of all manufacturers of marketed pharmaceuticals (MedSafe, 2022).
Lack of profitability is the main reason why many existing antibiotics are not available in some countries
In some cases, when generic antibiotics are being used in small quantities and the margins for profits are particularly low, market authorization holders may choose not to place a product on the market or to withdraw older antibiotics completely from specific markets. This means there are many so-called ‘forgotten’ antibiotics that have potential to treat resistant bacterial infections or to be used to save last-resort options for the most severe cases that are not available in many countries. In 2011, a survey of pharmacists, microbiologists and infectious disease experts from Europe, the US, Canada and Australia reviewed availability of 33 antibiotics, and discovered that 22 were available in fewer than 20 of 38 countries (Pulcini et al., 2012). A follow-up survey, conducted in 2015, revealed that 25 out of 36 selected antibiotics were available in 20 of 39 countries (Figure 11) (Pulcini et al., 2017). There have also been similar findings in low- and middle-income countries (LMICs) (Tebano et al., 2019). Limited profitability due to a combination of small market size and volume sales plus low prices, was the major reason for these antibiotics not being available in certain countries. The regulatory costs to keep antibiotics on the market, or to create new availability of antibiotics not previously registered in a country, were also barriers to maintaining access to antibiotics. Other factors contributing to withdrawals were lack of demand among clinicians and non-inclusion in national prescribing guidelines. However, there have been examples of professional groups lobbying government and national regulatory agencies to make older antibiotics available in their country. Examples include temocillin, and amikacin being marketed in France after being absent from the French market for several years following intervention by the French National Drug Agency (Pulcini et al., 2017; AFMPS, 2022).
4. What actions can be taken at the EU level to stimulate research, innovation and development of new antibiotics?
This chapter discusses what actions can be taken at the EU level to stimulate research, innovation and development of new antibiotics. First, we review different incentive mechanisms that can be deployed to achieve these aims including push incentives, such as research grants and tax incentives, and pull incentives, such as reimbursement reforms and advance purchase commitments. Second, we look at what progress has been made in implementing both push and pull incentives internationally. Third, we consider what principles should underpin the development of a holistic incentive package for antibiotic R&D. Finally, we consider the challenges that exist around implementation of the aforementioned incentives.
4.1. Push and pull incentive mechanisms to stimulate research and development
4.1.1. Overview of push and pull incentives
Push incentives reduce the cost of researching and developing new antibiotics, while pull incentives increase or ensure potential revenues once antibiotics reach market approval
Push and pull incentives are broadly used to classify the two main types of mechanisms for supporting antibiotic R&D (Table 5) (Renwick, Simpkin & Mossialos, 2016). Push incentives, such as research grants, tax incentives and R&D partnerships, reduce the cost of researching and developing new antibiotics (Morel & Mossialos, 2010). Whereas, pull incentives attempt to increase or ensure the potential revenue generated by antibiotics that successfully achieve market approval (Mrazek & Mossialos, 2003). This may be through direct financial pull incentives, such as milestone prizes, pay-for-performance agreements or advanced commitments to purchase the drug.
Table 5
A range of push and direct or indirect pull incentives can be used to stimulate antibiotic development.
Pull incentives can potentially ‘delink’ investments in R&D from sales volumes and price, which can create a viable market and also encourage stewardship
Depending upon their size and terms, pull incentives can potentially completely replace the revenue generated by new licensed antibiotics. This concept is referred to as ‘delinkage’, as the investment in R&D is delinked or decoupled from its sales volume and price (UN General Assembly, 2016 ; So et al 2011). This can achieve the dual aims of creating a fair return on investment for antibiotics and encouraging affordability and stewardship efforts by removing the incentive to increase price and sales volumes (Rex & Outterson, 2016). In addition to direct financial pull incentives, indirect financial pull incentives exist, such as accelerated access pathways that reduce the timeframes for regulatory assessment, or extensions of patent periods that provide companies with market exclusivity for longer periods. These are indirect financial incentives as they do not involve direct payments to antibiotic developers but instead provide financial rewards by increasing the revenue generated by new antibiotics. This terminology of direct and indirect financial pull incentives has been used in a recent policy document signed by several Member States (Austria, Belgium, Finland, France, Hungary, Ireland, Latvia, Lithuania, Luxembourg, Poland, Portugal, Slovakia, & Slovenia, 2022).
4.1.2. What progress has been made in implementing push incentives?
Major international and national programmes have been established to encourage antibiotic R&D
Over the last decade, government agencies, non-governmental organizations and drug developers have accelerated efforts aimed at encouraging antibiotic R&D through the formation of major international and national programmes (Figure 12). A review of programmes published in 2017 identified more than 58 different initiatives that incentivize the development of novel antibiotics, functioning at either the multilateral, EU or national level (Simpkin et al., 2017). In total, since the 2016 UN High Level Meeting on AMR there has been over $2bn invested in push incentives through multi-lateral, national, and private sector initiatives.
Multilateral-level initiatives include GARDP, CARB-X, JPIAMR and GAMRIF
At the multilateral level, key antibiotic R&D funding initiatives include the Global Antibiotic Research and Development Partnership (GARDP), the Combating Antibiotic Resistant Bacteria Biopharmaceutical Accelerator (CARB-X), the Joint Programming Initiative on Antibiotic Resistance (JPIAMR),4 and the Global Antimicrobial Resistance Innovation Fund (GAMRIF). There are also several international fora where antibiotic R&D is discussed, mapped and coordinated, including the Transatlantic Taskforce on Antibiotic Resistance (TATFAR), Global AMR R&D Hub, and G20 and G7 meetings.
EU-level initiatives include EDCTP and subsidiary programmes of IMI, and new initiatives such as IHI and HERA
At the EU level, major initiatives include the European and Developing Countries Clinical Trial Partnership (EDCTP), and subsidiary programmes of the public–private partnership called the Innovative Medicines Initiative (IMI), including the New Drugs for Bad Bugs (ND4BB) programme and the AMR Accelerator. The ND4BB programme ended in 2021 and had multiple streams, including: ENABLE and TRANSLOCATION focused on drug discovery and preclinical development; COMBACTE focused on drug development for Gram-positive bacteria; and COMBACTE-CARE, COMBACTE-MAGNET and IABC focused on drug development for Gram-negative bacteria (Kostyanev et al., 2016). Evaluation of the ND4BB and its multiple streams will be important to map enablers and barriers to incentivising antibiotic research and development encountered by these programmes. DRIVE-AB was focused around the economics and policy challenges of encouraging responsible use of antibiotics and incentivizing R&D. Some ND4BB initiatives have continued through other funding mechanisms; for example, ENABLE-2 was launched using funding from the Swedish government (Uppsala University, undated), and the European Clinical Research Alliance for Infectious Diseases (ECRAID) provides a platform for coordination of clinical development and is envisaged as a long-term successor to the COMBACTE programmes (ECRAID, undated). The AMR Accelerator was only launched in 2018 and continues with €489 million of funding allocated to nine projects with the aim of delivering 10 preclinical or clinical antibiotic candidates and five phase 2- or 3-ready assets (IMI AMR Accelerator, undated).
Under Horizon Europe, the new public–private partnership called the Innovative Health Initiative (IHI) Joint Undertaking has been launched, with a budget of €2.4 billion for 2021–27 (IMI, 2021). However, it is not clear to what extent IHI will invest in antibiotic R&D as research priorities are being defined throughout its implementation and IHI has a much broader scope than IMI. Alongside IHI, the European Commission has recently established the EU Health Emergency Preparedness and Response Authority (HERA), with a budget of approximately €6 billion for the years 2021–27. HERA will be responsible for several functions, including horizon scanning, funding R&D, supporting manufacturing capacity, and stockpiling (Anderson, Forman & Mossialos, 2021). Given a broader focus on pandemic preparedness, it is expected that only a small proportion of this 6 billion euro budget will be used to support R&D and access to antibiotics specifically.
Countries such as Germany, the UK, the USA and Sweden have made significant contributions to international and other initiatives focused on R&D
At the national level, the German government has made significant contributions to international initiatives such as the aforementioned CARB-X, GARDP and JPIAMR. The UK government has also contributed to CARB-X, as well as other initiatives such as GAMRIF and the Fleming Fund. The US government has invested significant funds through the Biomedical Advanced Research and Development Authority (BARDA) and the National Institutes of Health. There have also been substantial investments in antibiotic R&D by several EU Member States, for example the aforementioned investment by the Swedish government to launch the ENABLE-2 programme.
The AMR Action Fund and REPAIR Impact Fund are the key industry initiatives
There are also two major industry-led initiatives on antibiotic R&D. The industry-sponsored AMR Action Fund, established in 2020, makes equity investments in SMEs that are developing antibiotics that target priority pathogens. So far, in 10 years, it has raised US$1 billion in funding from industry, which will be targeted towards the clinical stages of development, with the aim of bringing two to four new antibiotics to market by 2030 (AMR Action Fund, 2022). The REPAIR Impact Fund was established by the Novo Nordisk Foundation in 2018, with a budget of US$165 million to invest in preclinical research for antibiotics that target priority pathogens. So far, it has invested about US$75 million across 10 projects to bring at least one new antibiotic to market (REPAIR Impact Fund, undated).
4.1.3. What progress has been made in implementing pull incentives?
Implementation of pull incentives has seen less progress compared to push incentives
There has been less progress in implementing pull incentives compared to push incentives for antibiotics, and this continues to be a major barrier to the creation of a viable market for antibiotics in many countries. A recent review on reimbursement models to incentivize antibiotic R&D identified practical applications of different pull incentives in France, Germany, Sweden, the UK and USA (Table 6). Moving forward, there needs to be evaluation of the different approaches to pull incentives used so far to establish to what extent they have improved access to antibiotics and stimulated innovation in research and development.
Table 6
The last decade saw the introduction of pull incentives in France, Germany, Sweden, the UK and USA, from which other countries can learn.
France introduced exceptions to the HTA criteria for new antibiotics and exclusions from the clawback mechanisms for pre-existing ones
In France, there have been exceptions to HTA criteria applied since 2021 that ensure new antibiotics are not reimbursed at prices lower than the lowest price across the UK, Germany, Italy and Spain, even if the HTA assessment classifies the new antibiotic as providing only minor benefits over pre-existing treatments. Other regulatory changes in France have been implemented to prevent shortages of antibiotics or withdrawals of antibiotic manufacturers from the French market. Drug shortages are a major issue in France, with alerts for drug shortages to the French drug agency (Agence nationale de sécurité du médicament et des produits de santé, ANSM) increasing 20-fold between 2008 and 2018 (WHO, 2021b). Antimicrobials, including antibiotics, represent 21% of all reported drug shortages in France (WHO, 2021b). These exclusion of generic and orphan antibiotics (alongside other generic and orphan drugs) from a clawback mechanism for pharmaceutical companies to repay some profits that have annual increases in revenue beyond a certain level, and the ability to increase prices paid for antibiotics with no other alternative available if a manufacturer informs the French drug agency that they plan to cease production or withdraw from the French market and demand is clearly documented and reported (CEPS, 2015).
Germany grants special treatment to antibiotics targeting priority pathogens
Similar to France, Germany has adjusted its HTA processes to account for how new antibiotics are typically approved using data from non-inferior clinical trials. This is important in Germany as new medicines are subject to internal reference pricing if additional clinical benefit cannot be demonstrated. Legislation was introduced in 2017 that allows resistance patterns to be considered when deciding if a new antibiotic is classified as providing additional clinical benefit and therefore not subject to internal reference pricing (Bundeministerium der Justiz, undated). In 2020, Germany introduced the Act on Fair Competition among the Statutory Health Insurance Funds (GKV-FKG), which included provisions to grant new antibiotics ‘reserve’ status if the antibiotic meets criteria such as targeting ‘priority pathogens’. If granted, antibiotic developers do not need to prove additional benefit of the new antibiotics, and can take advantage of early benefit assessment. In May 2022, cefiderocol (Fetcroja®) was the first antibiotic granted this ‘reserve’ status (Pownell, 2022).
In England, NICE uses subscription payments to incentivize the development of new antibiotics for resistant infections
In England, NICE and the National Health Service (NHS) England have implemented a subscription payment model to incentivize pharmaceutical companies to develop new antibiotics for resistant infections (HM Government, 2019). Following this, two pharmaceutical companies (Pfizer and Shionogi) submitted two novel antibiotics for assessment under the scheme. In April 2022, NICE and NHS England agreed on the terms of the first and second subscription contracts with these pharmaceutical companies to pay up to £10 million a year for access to two novel cephalosporin antibiotics, cefiderocol (Fetcroja®) and ceftazidime/avibactam (Zavicefta®). The initial agreement is for 3 years, with an option to extend up to 10 years (NICE, undated). Both antibiotics underwent a cost-effectiveness assessment and the contract value was set at an amount that would provide the pharmaceutical industry with an incentive to invest in antibiotic R&D, if other countries also agreed proportionate sums scaled to their proportion of global gross domestic product (GDP). If all G20 countries were to agree to pay subscription payments proportional to their GDP, this would result in payments of approximately £380 million per year for each new antibiotic (World Economics, 2022). This is broadly in line with estimates of the appropriate size of an international pull incentive to sufficiently stimulate innovation and research and development for new antibiotics (Outterson, 2021).
Sweden offers annual revenue guarantees to secure access to pre-existing and new antibiotics
In Sweden, the government has piloted a revenue guarantee model to secure access to pre-existing antibiotics of high medical value to counteract the fact that some newly approved antibiotics were not being launched in Sweden as it was not seen as economically viable by suppliers (Public Health Agency of Sweden, 2020). In July 2020, the procurement phase of the programme was complete and guaranteed annual payments of SEK4 million (approximately €400,000) per product were agreed for five antibiotics (Public Health Agency of Sweden, 2019). Of these, four were newly approved antibiotics, including ceftolozane/tazobactam (Zerbaxa®), imipenem/cilastatin/relebactam (Recarbrio®), cefiderocol (Fetroja®) and meropenem/vaborbactam (Vaborem®), plus one older antibiotic not previously marketed in Sweden, fosfomycin. The analysis of the initial pilot phase of this policy has been published, and has concluded that the policy was largely successful and has ensured that Sweden gained access to several new antibiotics and also earlier than other comparable European countries (Public Health Agency of Sweden, 2023).
In the USA, specially designated antibiotics can benefit from regulatory and financial incentives, while the introduction of subscription-style contracts is being debated
In the USA, there have been several pull incentives with minimal impact. In 2012, the Generating Antibiotic Incentives Now (GAIN) Act was brought in, allowing expedited regulatory assessment and an extension of market exclusivity of new antibiotics by 5 years if they are granted ‘qualified infections disease product’ (QIDP) designation (US Congress, 2011). To date, more than 100 QIDPs have been granted for antibiotics that target serious or life-threatening infections and drug-resistant pathogens (Outterson et al., 2022a). So far, the evidence suggests this policy has served to accelerate antibiotic candidates in the late stages of development only (Darrow & Kesselheim, 2020). Moreover, QIDPs were granted to any infectious disease product without any preference for new medicines or treatments that have novel characteristics or target clinical indications with higher health burden. In 2019, the Centers for Medicare and Medicaid Services (CMS) combined a financial incentive with the aforementioned regulatory incentive by allowing all new QIDP-designated antibiotics to be eligible for ‘new technology add-on payments’ (NTAPs) above and beyond standardized hospital payments for admissions with infections. This removed incentives to only use pre-existing generic antibiotics rather than new antibiotics, which were sometimes too costly. However, NTAPs have not addressed fundamental market failures such as small sales volumes and many antibiotic developers that have been granted NTAPs have still later declared bankruptcy or experienced significant economic losses. The Pioneering Antimicrobial Subscriptions to End Upsurging Resistance (PASTEUR) Act (originally introduced in 2020) is currently being debated in the US Congress and would establish a Committee on Critical Need Antimicrobials to grant a subscription-style contract with upfront payments to antibiotic developers with new antibiotics that address unmet need and add significant clinical value. The value of contracts would range from US$750 million to US$3 billion over 5 to 10 years depending on ‘favoured’ characteristics of the new antibiotic, such as having a novel target or mechanism of action and targeting priority pathogens, and funded with a budget of US$11 billion over 10 years (US Congress, 2020).
4.2. What principles should underpin the development of a holistic incentive package for antibiotic research and development?
A holistic package of push and pull incentives is needed to stimulate antibiotic R&D
There is consensus that no single incentive will be sufficient to stimulate antibiotic R&D, and an end-to-end approach is needed that applies a combination of push and pull incentives across the antibiotic R&D pathway. This has also been modelled (Outterson, 2021), and an appropriately sized pull incentive would predominantly function to incentivise development from Phase 2 or 3 clinical trails onwards, with push incentives required to simulate development from drug discovery to early clinical trials. Each incentive has advantages and disadvantages in terms of expected impact and operational feasibility. Therefore, a framework or set of principles is required to guide policy-makers when assessing the suitability of different options for incentives at the European level. Renwick, Simpkin & Mossialos (2016) developed such a framework, centred around three levels that examine public health, market and feasibility criteria (Figure 13).
4.2.1. Public health factors: medical needs, stewardship, access and environmental protection
Most importantly, a holistic incentive package for antibiotic R&D reinforces broader public health objectives. There are four key public health principles to consider:
Principle 1: Targeting high-priority medical need
Incentives should reflect the extent to which new antibiotics are expected to reduce the health burden of antibiotic-resistant bacterial infections
Antibiotic developers need to be remunerated in a manner that incentivizes innovation in antibiotics that target clinical indications of high-priority medical need. This means that incentive design should reward antibiotic developers proportionally according to the extent to which new antibiotics are expected to reduce the health burden of antibiotic-resistant bacterial infections. We know that over 80% of the health burden of antibiotic resistance in Europe is the result of bloodstream infections, intra-abdominal infections and respiratory tract infections (Figure 14). This is also an important consideration for policy-makers to ensure that public investment in push and pull incentives are prioritized for antibiotics of significant societal value. Innovation could be incentivized towards high-priority medical need by modifying lists of priority pathogens to signpost clinical indications with the highest health burden from antibiotic-resistant bacterial infections. This would signal to antibiotic developers which should be prioritized and which may be eligible for greater investment in push and pull incentive funding.
Incentives should also promote development of antibiotics with innovative characteristics
It is also important that new antibiotics are novel and demonstrate innovative characteristics, such as a new mechanism of action, target or chemical class. To incentivize innovation in a meaningful way, antibiotic developers need to be assured that reimbursement mechanisms will value these characteristics. This will require the application of novel approaches to HTA that consider additional elements of value specific to antibiotics, such as benefits from avoiding the spread of infection and enabling treatments in other therapeutic areas, such as oncology or surgical treatments (Box 3).
Principle 2: Promoting antibiotic sustainability
‘Delinking’ investments in antibiotic R&D from sales volumes and prices can support stewardship efforts
Antibiotic R&D efforts also need to consider how to promote antibiotic sustainability; in other words, to make sure that new antibiotics remain effective by limiting the emergence of resistant bacteria. This requires appropriate antibiotic stewardship and IPC. However, the traditional patent-based business model for medicines encourages pharmaceutical companies to increase sales to maximize return on investment before patent expiry. This typically conflicts with public health and stewardship objectives, as new antibiotics are often restricted for use in specific circumstances or as a second, third or even fourth treatment option. This is one of the major rationales behind ‘delinking’ investments in antibiotic R&D from sales volumes and prices when designing reimbursement mechanisms for antibiotics, as this removes the incentive to oversupply new antibiotics and also guarantees revenues for developers (Renwick, Brogan & Mossialos, 2016). There have been efforts from the regulatory perspective to promote stewardship of new antibiotics. For example, the EMA requires that developers include a statement on stewardship in the Summary of Product Characteristics for new antibiotics.5 It is also recommended that antibiotic developers produce stewardship and access plans (SAPs) for their products outlining what strategies they will deploy to ensure responsible stewardship. However, these access plans could also include emphasis on required IPC standards when using new antibiotics to avoid the spread of resistant bacterial strains. This is important as it is known that there is inconsistency in implementation of recommended IPC practices across Member States in Europe (Hansen et al., 2015; Zingg et al., 2015; Clack et al., 2018; Lacotte et al., 2020).
Principle 3: Improving patient access to new antibiotics
Incentive design needs to consider how to ensure consistent availability and access to new antibiotics for relevant populations
Even when new antibiotics have received regulatory approval, there is a substantial delay until reimbursement of up to 4 years in Member States of the EU (Figure 7). In many Member States, new antibiotics are simply not launched nor equitably accessible (see section 2 for an expanded discussion of access issues for antibiotics). The Stewardship & Access Plan (SAP) Development Guide has been produced by CARB-X to help antibiotic developers develop SAPs (CARB-X, 2021), which are a requirement for antibiotic developers that have received funding from CARB-X once their product enters phase 3 trials. The guide outlines four components of access that are relevant to antibiotics (Box 5): first, whether the antibiotic is registered and accessible by patients; second, whether the supply of antibiotic is consistent and available to patients and healthcare professionals in practice; third, whether the antibiotic is affordable and suitable reimbursement mechanisms are in place; finally, the acceptability of the antibiotic to the public and the inclusion of relevant health promotion and educational efforts to ensure access and appropriate use.
Box 5
The CARB-X guide outlines four components for access to antibiotics.
Principle 4: Protecting environmental health
Incentive design should ensure that manufacturers and suppliers abide by relevant environmental standards
The dissemination of antibiotics in the environment can further drive the spread and development of resistant bacteria (Booton et al., 2021). The AMR Industry Alliance Manufacturing Framework has produced guidelines for monitoring antibiotic concentration in manufacturing waste against set concentration limits (AMR Industry Alliance, 2019), and the Indian government also attempted to introduce regulation in 2020 to enforce concentration limits for antibiotic levels in effluent wastewater (ReAct, 2020a). To ensure compliance with these terms, incentive design should include mandatory requirements for suppliers to abide by these standards and manufacture antibiotics in a manner that limits their emissions to the environment. This will also include procurement of reaction intermediates and APIs from suppliers that commit to internationally agreed standards for antibiotic levels in manufacturing effluent (BCG & Wellcome Trust, 2021). Once antibiotics achieve market approval, reimbursement and tendering processes should also include mandatory requirements for suppliers to demonstrate they have complied with these environmental health standards. However, implementation of such as policy will include increases in prices for generic antibiotics and investment in the necessary personnel and infrastructure to monitor compliance.
4.2.2. Market enablement factors: creating a financially viable market for antibiotics
The economic or market factors of incentives for antibiotic R&D include the following two principles:
Principle 1: Ensuring sufficient return on investment from developing and commercializing a new antibiotic
Incentives need to either reduce the costs or risk of investments in R&D and/or increase revenues from new antibiotics, but there are uncertainties about their appropriate size
The relative expected NPV of antibiotics in comparison with other therapeutic areas has contributed to most large pharmaceutical companies withdrawing from investing in antibiotic R&D. To improve the expected NPV of antibiotics, incentives need to be designed that either reduce the costs or risk of investments in R&D and/or increase revenues from new antibiotics. This will require public investment in push incentives for basic science research and preclinical development when the risk of failure is particularly high, and public investment in pull incentives that increase expected revenues for new antibiotics. In turn, this will make private investment in clinical antibiotic R&D a more attractive prospect, and hopefully stimulate innovation.
There have been studies of the appropriate size of a global ‘pull’ incentive needed in order to be effective. For example, several analyses indicate that a market entry reward (MER) of approximately US$1 billion per antibiotic globally, in addition to unit sales revenues, would be sufficient (Review on Antimicrobial Resistance, 2016; Stern et al., 2017; Årdal et al., 2018) In 2018, DRIVE-AB estimated that this size of incentive could lead to the delivery of approximately 16–20 new antibiotics over 30 years (Årdal et al., 2018). However, another analysis has estimated that the size of a partially delinked pull incentive (MER in addition to unit sales) would need to be between US$1.5 and US$2.2 billion to be effective, and a fully delinked pull incentive (subscription-style model over 10 years) would need to be between US$3.3 and US$8.9 billion to be effective (Outterson, 2021). However, these analyses are sensitive to uncertainties around factors such as transition probabilities between different stages of developments, the costs of antibiotic R&D, and expected sales volumes and unit prices.
Principle 2: Make market participation feasible for SMEs
Incentives should be designed to support SMEs during the preclinical and early clinical stages of development through push funding
As shown in Figure 5, most of the innovation in antibiotic R&D is conducted by SMEs, many of which have promising antibiotic candidates but struggle to bring their compounds to preclinical studies and early stages of clinical development. This is because SMEs have significantly smaller capital reserves than large pharmaceutical companies and cannot fund the necessary costs of preclinical studies (estimated in one study to be between US$23.5–26.5 million in 2021) and phase 1 trials (estimated to be between US$19.8–28.8 million in 2021) (Outterson, 2021). Securing outside investment is also particularly challenging during these phases, as the probability of success is much lower than in the later stages of clinical development (Figure 4). Therefore, incentive packages to stimulate antibiotic R&D need to carefully consider how to support SMEs during the preclinical and early clinical stages of development through push funding to allow them to bring promising antibiotic candidates to the more favourable late-stage clinical trials.
4.2.3. Implementation, sustainability and operational feasibility: ensuring a global end-to-end approach to antibiotic R&D
Effective implementation and operationalization of incentive packages requires careful consideration of potential political, regulatory, legislative, industry and financial hurdles
When designing an incentive package for antibiotic R&D, it is also important to consider the political, regulatory, legislative, industry and financial hurdles that may be faced during implementation. In Europe, there are particular challenges in navigating the complexities of EU legislation. For example, the boundaries between the scope of EU- and national-level health policies sometimes create tensions, as pre-existing treaties such as the Treaty on the Functioning of the European Union (TFEU) state that the EU must respect Member States’ autonomy in operating their own health systems (Mossialos et al., 2010a). As the purpose of this policy brief is to provide an introduction to some of the relevant challenges and policy responses to securing sustainable access to pre-existing and new antibiotics only, a full exploration of the legislative feasibility of different incentives for antibiotic R&D should be the subject of future research.
Different incentives also have varying financial implications for the European Commission and Member States, depending upon to what extent the European Commission shares the burden of financially contributing to different incentives with Member States. For incentives that are implemented at the EU level but financed by Member States, there would need to be an institution, such as the European Investment Bank (EIB) or HERA, responsible for coordinating or collecting proportional financial contributions. Issues such as deciding by what metric proportional payments are determined, and securing collective agreement among Member States on the size of incentive, could prove controversial.
Governance of the incentive package can be aided by having an effective global ‘antibiotic pipeline coordinator’
Governance of the incentive package is also important as there needs to be transparency regarding the methodological assumptions and rationale behind the design (including size of the incentives), and implementation at different stages of the antibiotic R&D pathway. This will involve broad consultations with patient, health professional, industry, academic and government stakeholders and representatives. The societal value and likely success of alternative antibiotic candidates will also need to be modelled to ensure that investments are optimally distributed. The aim should be to achieve an end-to-end approach to antibiotic R&D, with full oversight of where additional investment or technical and scientific support are required. Achieving this will require designating responsibility for assessing antibiotic candidates to a scientific committee or establishing an ‘antibiotic pipeline coordinator’.
An ‘antibiotic pipeline coordinator’ is a governmental or non-profit organization that has responsibility of oversight of the antibiotic pipeline, including identifying unmet needs and actively supporting R&D projects to address these unmet needs (Baraldi et al., 2018). While the Global AMR R&D Hub produces descriptive analyses of antibiotic R&D projects, an effective ‘antibiotic pipeline coordinator’ needs to use multiple forms of financing, invest across all development stages, and provide a platform to share technical support and expertise among antibiotic developers (Wellcome Trust, 2016; Glover, Knight & Chandler, 2021). The latter point is particularly important in the earlier stages of preclinical and clinical development, as SMEs often do not have the scale necessary to achieve this expertise internally. In addition, an ‘antibiotic pipeline coordinator’ could support clinical trial networks such as the European Clinical Research Alliance on Infectious Diseases (ECRAID) (ECRAID, undated), ADVANcing Clinical Evidence in Infectious Diseases (ADVANCE-ID) (ADVANCE-ID, 2022) and the Global Health European and Developing Countries Clinical Trials Partnership (GH EDCTP3 Joint Undertaking) (EDCTP, undated). The ENABLE platform did fulfil some of these pipeline coordinating functions for a portfolio of push incentives in early stages of development, until it ended at full capacity in October 2021. As mentioned above, there is now a cut-down version of it supported by the Swedish Government, ENABLE-2, with very limited and short-term resources. Internationally, BARDA, CARB-X, and GARDP fulfil many functions at different stages of development (Table 7).
Table 7
CARB-X, BARDA and GARDP fulfil many functions of pipeline coordinators.
Optimal deployment of push and pull incentives requires a global solution
It is important that any efforts to strengthen incentives for antibiotic R&D at the EU level engage with other international efforts, as optimal deployment of push and pull incentives are likely to require a global solution including initial implementation by at least all G7 countries, and later expansion to G20 countries. Strengthening international coordination should encourage sharing information, resources and expertise, facilitating cooperation to meet public health goals, streamlining the supply chain and improving regulatory transparency. Collectively, this would indirectly reduce the cost of antibiotic R&D and align priorities among different public and private stakeholders.
4.3. What policy options can be considered by European policy-makers to strengthen research and development of novel antibiotics?
A number of policy options to strengthen R&D of novel antibiotics has attracted policy attention at the European level
Drawing upon stakeholder interviews conducted with policy-makers, academics and industry representatives (see ‘Stakeholder interviewees’ in the Prelim pages), suggestions from reference group members, and relevant literature referenced in this policy brief, a number of policy options for strengthening incentives for antibiotic R&D were identified for further analysis. Of the seven policy options analysed, one has been the topic of several recent policy papers (transferable exclusivity extensions, TEE) (Berdud et al., 2019; Anderson, Wouters & Mossialos, 2022; Boyer, Kroetsch & Ridley, 2022; EFPIA, 2022; EPHA & ReAct, 2022; Medicines for Europe, 2022; Wilsdon, Robson & Lu, 2022); three were referenced in a recent letter from Member States to the European Commission (MERs, subscription payments and milestone payments) (Austria, Belgium, Finland, France, Hungary, Ireland, Latvia, Lithuania, Luxembourg, Poland, Portugal, Slovakia, & Slovenia, 2022); one is already in use in the United States (market exclusivity extensions, MEE) (Darrow & Kesselheim, 2020); and one was previously proposed to the 2009 Swedish presidency of the EU (Options Model for Antibiotics) (Mossialos et al., 2010b). These selected policy options can be classified according to whether they are direct financial pull incentives (subscription payments, MERs); indirect financial pull incentives (TEEs, MEEs); or other financial incentives (milestone payments, Options Model For Antibiotics). Each policy option is considered drawing upon the aforementioned principles for antibiotic R&D (Table 8). In addition, we draw upon data and analysis shared by CARB-X to identify where gaps may exist for funding allocations across the antibiotic R&D pathway (Figure 17).
Table 8
Selected policy options for incentivizing antibiotic R&D.
There are many other incentives for antibiotic R&D that were not selected for further analysis in this policy brief, but are analysed in other studies (Renwick, Brogan & Mossialos, 2016; Årdal et al., 2018). In particular, three further options (Non-profit Mechanism for Innovative Antibiotic Development (MIAD), the ‘Pay or Play’ Model, and priority review vouchers) were also considered for inclusion but are instead discussed in ‘Supplementary material: Additional policy options’.
4.3.1. Direct financial pull incentives
Subscription payments
Subscription payments can fully or partially separate revenues from sales volumes, which supports stewardship and promotes patient access
Subscription payment models are a type of direct financial pull incentive that either pays the developer a fixed price for access to antibiotics upfront on an annual basis (as in the UK), or guarantees annual revenues (as in Sweden) (Boluarte & Schulze, 2022). Payments can vary proportionally depending on the extent of new antibiotic target priority pathogens or clinical indications with a significant health burden. Subscription payment models separate (or partially separate in the case of guaranteed annual revenues) revenues from sales volumes. This reduces incentives to increase volumes of sales, which can be harmful to stewardship efforts (i.e., antibiotic sustainability), and promotes patient access as subscription payments include requirements to provide antibiotics. It is also possible for these access agreements to include conditions on environmental health manufacturing standards.
The extent to which subscription payments influence antibiotic R&D depends on the level of annual payments, but they are unlikely to stimulate early development unless explicit signals are included
The influence of subscription payment models on antibiotic R&D is uncertain, and ultimately very dependent upon the level of yearly payments. If yearly payments are set to reflect the marginal cost of production of new antibiotics, they will have little impact on encouraging antibiotic R&D. If, however, they reflect the R&D costs of antibiotics and the risk of failure at different stages of development, yearly payments are more likely to encourage antibiotic R&D. There have been several estimates, ranging from US$1–8.9 billion, of the appropriate size of a global pull incentive required to stimulate antibiotic R&D (Review on Antimicrobial Resistance, 2016; Stern et al., 2017; Årdal et al., 2018; Outterson, 2021), although there is uncertainty regarding these costs and transition probabilities exacerbated by lack of transparency in the pharmaceutical industry. As subscription payment models are typically agreed towards the end of antibiotic development they will only have a substantial impact if the institution responsible for assessing antibiotic candidates and awarding contracts also provides reliable signals to antibiotic developers during the early stages of development that they may be eligible for subscription payments upon market approval (through a mechanism of ‘pre-qualification’).
An EU-level subscription model has been proposed
Implementation feasibility at the EU level may be dependent upon achieving consensus among Member States regarding the size of payments, the public health value of the new antibiotic, and sales volumes required to meet demand. EU-JAMRAI has proposed a mechanism, modelled on the Swedish approach to subscription payments for antibiotics, for a partially delinked subscription model for antibiotics which can be implemented at the EU level (Box 6).
Box 6
JAMRAI has proposed a mechanism for a partially delinked subscription model at the EU level.
Market entry rewards
MERs could be conditional on access agreements, or granted in exchange for intellectual property rights to facilitate earlier generic market entry
Market entry rewards are another direct financial pull incentive that functions by providing a one-off reward to antibiotic developers once they have successfully achieved regulatory approval. The size of MER might vary according to the extent to which a new antibiotic targets high-priority medical needs, for example there could be higher rewards for priority pathogens and clinical indications associated with higher health burden. An MER could be granted in exchange for access to a new antibiotic over a specified timeframe, or the MER could be granted in addition to marginal revenues for unit sales of antibiotics. The second option creates less incentive for antibiotic sustainability, as companies may still choose to oversell antibiotics and this may harm stewardship efforts (i.e., antibiotic sustainability). Moreover, even if MERs include terms and conditions for access to new antibiotics, they do not guarantee long-term access, for instance if an antibiotic developer does not comply with access agreements or subsequently becomes bankrupt. This could happen, as MERs do not address fundamental market failures for antibiotics (i.e., low sales volumes and unit prices). However, an appropriately sized MER could be granted in exchange for IP rights, and this could improve patient access as it would facilitate earlier generic market entry.
MERs may disproportionately benefit large pharmaceutical companies
MERs can be used to fund post-approval costs related to pharmacovigilance or commercialization (Wouters, McKee & Luyten, 2020), which is important as these costs have contributed to several antibiotic developers either becoming bankrupt or experiencing significant economic losses (ReAct, 2021). MERs can benefit SMEs if they retain the IP rights during regulatory approval but may disproportionately benefit large pharmaceutical companies as they often purchase the IP rights of medicines at later stages of development.
If they are implemented as a one-off reward, MERs could lead to significant short-term costs for Member States
Operationally, MERs would be feasible to implement at the EU level as they are a one-off reward, which could be granted following regulatory approval by the EMA. However, collecting proportional payments from Member States to finance MERs may be challenging, and if multiple MERs were granted at once this could be responsible for significant short-term costs. In contrast, the financial burden of subscription payments could be spread over multiple years. However, it should be noted that the costs of MERs can be spread over multiple years; for example, DRIVE-AB recommended that the financial costs of MERs should be spread over up to 5 years (Årdal et al., 2018).
4.3.2. Indirect financial pull incentives
Transferable exclusivity extensions
The pharmaceutical industry are supportive of TEEs as they would be easy to implement, do not require upfront government funding, and would provide a large incentive
Transferable exclusivity extensions, a type of indirect financial pull incentive, are a reward granted to antibiotic developers that successfully develop and launch a new antibiotic (EPHA & ReAct, 2022). They can be used to extend the regulatory data protection or IP rights of any medicine for a fixed time period (i.e., up to 12 months).6 Antibiotic developers could choose to use the voucher for one of their own medicines or auction it for the highest price if they do not have any drug with high sale potential. To maximize value, TEEs are likely to be applied to drugs with high annual revenues (Outterson, 2007). The pharmaceutical industry has been supportive of TEEs, emphasizing that they are easy to implement, do not require upfront government funding, provide a large enough incentive to be effective, and benefit pharmaceutical companies of all sizes (EFPIA, 2022). The length of a TEE could also be varied to link the public health value of new antibiotics to the size of reward received (Outterson and McDonnell 2016).
The exact impact of TEEs on public healthcare budgets is difficult to establish
Non-industry commentators have raised a number of important concerns around TEEs (Boyer, Kroetsch & Ridley, 2022; EPHA & ReAct, 2022; Medicines for Europe, 2022). First, the exact impact of TEEs on public healthcare budgets is difficult to establish, since any estimate is sensitive to assumptions about the impact of the generic market entry on prices and approach towards exclusivity extension. Årdal et al. estimate that the costs to European healthcare systems for a single TEE could be over €3 billion (Årdal, Lacotte & Ploy, 2020), whereas other industry sponsored analyses suggest it could be significantly less than €1 billion (Berdud et al., 2019; Medicines for Europe, 2022). However, none of these analyses distinguished between drugs eligible for longer market exclusivity if TEEs are granted for regulatory data protection or IP rights. This is important, as the method of exclusivity extension will determine which medicines are eligible for a TEE, as well as the potential financial impact on Member States’ pharmaceutical budgets (Copenhagen Economics, 2018). Therefore, further analysis is needed to establish the financial implications of these different methods of exclusivity extension.
It is not certain to what extent and at what stage TEEs would incentivize antibiotic R&D
Second, it is not certain to what extent and at what stage TEEs would incentivize antibiotic R&D. Evaluations of priority review vouchers for therapeutic areas with unmet need in the USA (Meyer, 2021; Aerts et al., 2022) found minimal impact on R&D. Instead, they functioned to bring forward supply of medications already in the late stages of development. Third, TEEs would need to include strict conditions to ensure access to and stewardship of antibiotics in all relevant markets. Otherwise, there is no guarantee that antibiotics will be launched consistently across Europe. Similar to MERs, TEEs do not address fundamental market failures for antibiotics (i.e., low sales volumes and unit prices). Therefore, there are still significant risks that the antibiotic developer may subsequently declare bankruptcy if the value of the TEE does not recoup antibiotic R&D costs. Unless TEEs are combined with prospective purchase agreements that delink sales and volumes, the antibiotic developer may still choose to oversell new antibiotics to maximize annual revenues. Fourth, TEEs do not link the public health value of new antibiotics to the size of reward received. This is because the value of a TEE is determined by which expensive, high-selling brand-name medicines are nearing patent expiry when it is granted, rather than whether an antibiotic displays any innovative characteristics or to what extent it addresses high-priority medical need. However, the European Commission could grant TEEs only to new antibiotics which display innovative characteristics in order to limit the possibility that TEEs are granted to antibiotics with limited public health value.
Market exclusivity extensions
MEEs are unlikely to have a substantial impact on total expected NPV for antibiotic R&D
Market exclusivity extensions, another form of indirect financial pull incentive, reward antibiotic developers that successfully develop and launch a new antibiotic by extending the IP rights of the new antibiotic for a fixed time period (i.e., up to 5 years). This prolongs the time period that antibiotic developers have market monopoly for their new antibiotic, and therefore increases the total revenues they can expect following launch of their new antibiotic. However, MEEs will likely have little impact on the profitability of commercializing new antibiotics as they do not address fundamental market failures for antibiotics (i.e., small sale volumes and low prices). Therefore, even with 5 years of additional market exclusivity, MEEs are unlikely to have a substantial impact on total expected NPV for antibiotic R&D. Although, if a new antibiotic were developed with several innovative characteristics, such as new mechanism of action, offering a significant improvement over pre-existing therapies, then an antibiotic developer might be able to justify higher prices as there would be limited competition with pre-existing generic antibiotics for the same clinical indication. In this so far unrealized scenario, an MEE could be responsible for the generation of significant additional revenue for the antibiotic developer. Similar to TEEs, unless MEEs include strict terms and conditions for antibiotic access and sustainability, they do not create incentives for access to and stewardship of new antibiotics, as they are one-off reward.
MEEs could negatively impact patient access to antibiotics as they would delay entry of generic manufacturers
In fact, MEEs could negatively impact access to antibiotics as the entry of generic manufacturers often results in improved access and some diversification of the manufacturing supply. While the length of MEE granted could vary to reward more highly innovative characteristics (such as a new chemical class or mechanism of action, or clinical indications with high health burden), this risks creating conditions whereby access to new antibiotics with high public health value is limited. Over the last decade, MEEs have been used in the USA following the implementation of the Generating Antibiotic Incentives Now (GAIN) Act 2012. However, this has been largely ineffective and many antibiotic developers that have been granted MEEs have subsequently gone bankrupt or suffered significant economic losses (Darrow & Kesselheim, 2020).
4.3.3. Other financial incentives
Milestone payments
Milestone payments may help SMEs to overcome the ‘valley of death’ for antibiotics in the early stages of development
Milestone payments are a type of push incentive that involves granting funding to developers at specific stages of antibiotic development. This funding is then used to fund subsequent stages of development. Milestone payments could be used to support SMEs to overcome what has been termed the ‘valley of death’, which results in the failure of many antibiotic candidates during preclinical and early stages of clinical development. They will have less impact on larger pharmaceutical companies, which are typically less involved in antibiotic development during the preclinical stages of development. Baraldi et al. demonstrate through simulations of thousands of ‘synthetic’ antibiotic R&D projects using literature on transition probabilities and R&D costs that milestone payments can have a significant impact on the expected NPV when granted following completion of phase 1 trials, which is typically when antibiotic developers (most of which are SMEs) are most financially vulnerable as earlier push incentive funding (such as grants) has become depleted (Baraldi et al., 2019). At this stage, the expected NPV of an antibiotic R&D project is very low and impacted less by pull incentives that are triggered at later stages of clinical development.
A global ‘antibiotic pipeline coordinator’ would need to take responsibility for assessing eligibility for milestone payments
Milestone payments can be targeted towards antibiotic candidates that address high-priority medical need. Assessing eligibility for milestone payments would need to be undertaken by an ‘antibiotic pipeline coordinator’, which could also facilitate cooperation among relevant stakeholders across the antibiotic pipeline (discussed further above). Ideally, this ‘antibiotic pipeline coordinator’ would function at the global level. There are already some organizations performing the functions of an ‘antibiotic pipeline coordinator’ at different stages of the antibiotic R&D pathway (Table 7). Such an antibiotic coordinator would also be tasked with interpreting the reliability and validity of clinical trial data, and would need to ensure that issuing milestone payments is dependent upon complete transparency regarding clinical trial findings.
One drawback of milestone payments is that there would be no guarantee that antibiotic candidates would successfully achieve market approval
A major advantage is that milestone payments are generally smaller in size and thus do not require the same large upfront investments as other pull incentives granted at the end of the R&D pathway, such as MERs or subscription payments. Moreover, the aforementioned simulations by Baraldia et al. indicate that milestone payments granted following the completion of phase 1 trials would require less funding than these pull incentives to successfully bring an antibiotic candidate through to market approval (Baraldi et al., 2019). However, the drawback of milestone payments is that there would be no guarantee that antibiotic candidates would successfully achieve market approval whereas the other pull incentives would only be granted once market approval had been achieved. Market payments can also, by design, include stewardship, patient access and environmental health agreements, but these stipulations are challenging to enforce as milestone payments are made during multiple stages of development and the ownership of IP rights for antibiotic candidates may change throughout the development process. However, this is also the case for other incentives discussed in this policy unless they are combined with some form of prospective payment agreement, such as subscription payments, with continued payments made conditional on compliance with these agreements.
Options Model for Antibiotics
The Options Model for Antibiotics (OMA) allows public investors to share the risk of investing in antibiotic development with antibiotic developers in return for access agreements and discounted prices
The OMA outlines a mechanism whereby public investors effectively become co-investors with antibiotic developers, sharing the risk of investing in antibiotic development in return for guarantees to access and discounted prices. Public investors can target investments towards antibiotic candidates that address high-priority medical need, and prospective purchase agreements can include conditions on access, antibiotic sustainability and environmental health manufacturing standards. This is a hybrid mechanism, as it combines push incentives (i.e., funding for early drug discovery research and early clinical trials) and pull incentives (i.e., advanced commitments to purchase antibiotics if they achieve market authorization). The OMA is modelled on the principle of financial call options and allows payers to invest in antibiotic R&D by purchasing the right to buy antibiotics at discounted prices if and when these products obtain market approval (Figure 16) (Brogan & Mossialos, 2013; Forman et al 2020; Brogan & Mossialos, 2016; Brogan & Mossialos, 2006). This effectively means that public and private investors share the risk of investing in antibiotics. Early investments in phase 1 trial (which have a higher probability of failure and therefore are riskier) options would be cheaper; while less risky options purchased towards the end of the antibiotic development cycle would be more expensive. As a co-investor, the options purchaser may potentially have the opportunity to codetermine the market price for the new antibiotic alongside the developer. Thus, the option purchaser benefits in several ways. Firstly, the options holder invests in, and therefore incentivizes, the development of the antibiotics they desire (i.e., public investors can choose antibiotics that meet target drug profiles and unmet needs). Second, the options holder becomes a co-investor, and can therefore exert influence over price settings if and when that antibiotic successfully receives market approval. Thirdly, the options holder can ensure access to the antibiotic at a discounted price and in specified quantities (Brogan & Mossialos, 2013).
Implementation of the OMA would require a legislative framework to regulate agreements between option sellers and purchasers
There are some practical considerations for implementing the OMA. First, a legislative framework would need to be created that would provide antibiotic developers (option sellers) and investors (public purchasers) with confidence that options agreements would be adhered to, including designating roles and responsibilities to relevant institutions for monitoring compliance and resolving any potential disagreements between antibiotic developers and investors. Second, estimating the appropriate size of investments and discounts on yet-to-be determined future prices might be challenging to calculate. During the early stages of development, antibiotic developers might be reluctant to share the necessary data and clinical trial findings required to undertake these calculations. However, this could be overcome with confidentiality agreements. Third, the uncertainties regarding the potential effectiveness of new antibiotics at earlier stages of development may discourage investments following the completion of preclinical or phase 1 trials when bottlenecks in antibiotic R&D exist. Investments would be cheaper during these earlier stages of development to reflect the risk of investment, but they would also need to be large enough to significantly contribute to funding the next stage of development.
4.3.4. The case for greater push incentive funding
There is a funding gap for preclinical and earlier stages of clinical development, which contributes to many antibiotic candidates not continuing to later stages of development
While there has been substantial investment in push incentives over the last decade (see section 2), as of 2023, the majority of expected investments in antibacterial therapeutic R&D over the coming decade is either concentrated in the basic science research or the later stages of advanced clinical development. This means there remains a very significant funding gap for early-stage product development, a period during which SMEs are particularly vulnerable to experiencing financial difficulties. Figure 17 shows the results of a preliminary analysis, which projects various public and private funding allocations for antibacterial therapeutic R&D over the next decade, drawing upon data from the Dynamic Dashboard of the Global AMR R&D Hub and expected investments from CARB-X, the AMR Action Fund, G7 countries and HERA, among others. This is compared to the minimal investments required over a decade to bring to market at least six novel antibacterial treatments, using drug development costs and transition probabilities identified from a literature review by Outterson (2021).
While it should be acknowledged that there are uncertainties regarding transition probabilities and antibiotic development costs, this preliminary analysis does demonstrate the significant funding gaps during the early stages of product development, which are likely to be responsible for many antibiotic candidates not continuing to later clinical stages of development. The full assumptions underlying this analysis are included in Supplementary Tables 1 and 2. The largest uncertainty is for transition probabilities from basic science and drug discovery research to preclinical trial phases, as no reliable estimates exist. Instead, this is reliant on expert opinion.
As discussed above, the conclusion of the ENABLE research programme points to the even greater unmet need for push incentives from the European Commission, such as direct funding and grants during early stages of antibiotic R&D, especially in the transition from early drug discovery to preclinical development. This is even more urgent and timely in order to take advantage of the creation of the AMR Action Fund and its planned injection of US$1 billion of private investment in clinical development until 2032. Drawing upon the analysis above, there is a strong argument for the EU to also consider increasing funding for push incentives alongside implementing pull incentives, as well as to join other G7 governments in supporting antibiotic pipeline coordinators to distribute this push incentive funding with responsibility for oversight of antibiotic candidates under development, identification of unmet needs for funding, and the provision of platforms to share technical support and expertise among antibiotic developers (see section above on implementation and operational feasibility).
5. How can the EU promote sustainable and timely access to pre-existing and new antibiotics once developed and approved?
Most antibiotic-resistant infections would respond to antibiotics that have already been developed and approved for market, but not all existing antibiotics are reliably available in all countries
Timely access to effective treatments for infections is crucial for patients and for achieving the best possible clinical outcomes. It requires not only focusing on the development of new antibiotics, as described in previous sections, but also ensuring that existing effective options are readily available when needed. Indeed, despite the persistent and expanding challenge of antibiotic resistance, most antibiotic-resistant infections would respond to antibiotics that have already been developed and approved for market. However, not all existing antibiotics are reliably available in all countries and this further drives resistance development due to the use of less effective and/or alternatives with a broader spectrum of activity (see section 3). Therefore, it has become clear that ensuring access to and availability of existing antibiotics is a key component in tackling antibiotic resistance in addition to the primary function of treating patients and saving lives.
Several factors influence the availability of an antibiotic in a given country, including whether manufacturers have brought or kept their product on the market, and whether there is sufficient supply of the antibiotic overall. The following paragraphs explore these parameters and present selected approaches that might facilitate better availability in the future, focusing on action at the EU level; these approaches span regulatory/administrative measures, measures related to pricing and procurement, and measures to better understand and strengthen the antibiotics supply chain. It is important to note that these dimensions are interlinked and should therefore be considered in combination, just as previous sections have showcased for the development of new antibiotics. In line with Table 8, which summarizes options to facilitate the development of new antibiotics, Table 10 at the end of this section summarizes the main options presented below; it uses the same criteria, adapted for applicability.
Reducing registration fees and adapting sunset clauses can lower administrative hurdles that contribute to the variable availability of existing antibiotics across countries
Cross-country comparisons of registered antibiotics show considerable variability in the number and composition of antibiotic products available on the market (Pulcini et al., 2012, 2017; Tebano et al., 2019). This may reflect that marketing authorization holders choose not to register a product in a given country in the first place, or that they have opted to withdraw after a while. Financial considerations, and in particular the expectation of insufficient revenues, are cited as the main reason for such decisions (Boluarte & Schulze, 2022). The constituent drivers of this market failure are described in detail in section 3 above, and comprise relatively small target volumes (given epidemiological trends and conservation efforts), brief treatment regimens, and low prices (particularly for older antibiotics with generic competition). Previous analyses, including by the Swedish Dental and Pharmaceutical Benefits Agency (TLV), show a direct relationship between sales value and a pharmaceutical product disappearing from the market (European Commission et al., 2021; Dental and Pharmaceutical Benefits Agency et al., 2022). For the Swedish context it was estimated that an annual revenue below SEK 1 million (ca. €92,000) increased the likelihood of deregistration (see Figure 18).
Figure 18
The example of Sweden shows a direct relationship between sales value and proportion of products that disappear from the market. Source: Dental and Pharmaceutical Benefits Agency et al., 2022.
Market authorization holders may opt to withdraw their products to avoid annual fees, especially if these exceed the expected product revenues (for reference, annual fees for centrally authorized medicines in the EU exceed €100,000) (EMA, 2022a). At the same time, should they decide to bring the product to market again after withdrawal, additional administrative costs can be expected. If market authorization holders choose not to deploy their products in certain countries without actively seeking to withdraw marketing authorization, regulators at the national level may have legitimate expectations for sufficient volumes to be made available, and so-called ‘sunset clauses’ are activated as per EU or national law (see Box 7), potentially triggering an additional financial and administrative burden. Since the establishment of the EMA’s centralized marketing authorization procedure in 2005, antibiotics are typically reviewed under this pathway, and the decision is binding for all countries in the EEA; however, given that many older antibiotics received their marketing authorization before 2005, there is variability among countries particularly for this group of medicines (Årdal et al., 2017).
Box 7
The ‘sunset clause’ for medicines in Europe can trigger an additional financial and administrative burden for market authorization holders.
Potential regulatory policy options to discourage the withdrawal of existing antibiotics include, among others, the reduction or abolition of annual fees and adapting sunset clause requirements (see Box 7). This has already been suggested in Sweden, as part of an initiative to ensure that existing antibiotics stay on the market (Dental and Pharmaceutical Benefits Agency et al., 2022). At the EU level, similar considerations might be levied for antibiotics with a central marketing authorization by the EMA, and communication with Member States could reinforce the potential for applying related options at the national level. Such actions could apply to all antibiotics, or be combined with clear criteria regarding aspects of public health importance (e.g. linked to effectiveness) and expected sales values that would trigger exceptions from annual fees and sunset clauses. The latter option takes into account that there are other therapeutic areas, such as rare diseases, which may be eligible for similar benefits. In either case, fees for other therapeutic areas may also need to be adjusted to ensure financial sustainability of regulatory agencies. Other options to lower the threshold for the (re)registration of antibiotics may include allowing (re)registration based on older documentation, fast-tracking registration processes for antibiotics based on resistance metrics, and harmonizing packaging and leaflet information (Dental and Pharmaceutical Benefits Agency et al., 2022). For the latter two options, it might be easier to implement this regionally (e.g., for groups of countries, such as the Nordics) (Dental and Pharmaceutical Benefits Agency et al., 2022) but it is also being discussed at the EU level (European Commission, 2022b).
While reducing administrative hurdles for antibiotics as described here might require relatively low-threshold changes that could contribute to the sustained accessibility of existing antibiotics, the effectiveness of such measures in counteracting current market failures would likely be limited in isolation, as it does not directly affect the size of expected revenue from sales (see also Table 9).
Table 9
Virtual stockpiling could be combined with public sector and private mandatory inventories.
Good procurement practice, joint purchasing and subscription payments are policies that can ensure sufficient revenues for antibiotic manufacturers and would facilitate access to essential generic antibiotics
The established paradigms of pharmaceutical procurement generally aim to minimize costs for public payers. This means that prices for generic medicines are generally low (e.g., due to internal reference pricing measures in the outpatient setting or lowest-price-based tenders in the inpatient setting), and that only a few suppliers are contracted for the delivery of these medicines (e.g., because of single-award tenders in the inpatient setting). Previous sections have established the importance of sufficient revenues and supply-chain diversification for sustained access to existing antibiotics. Given the size of their target populations, the short durations of treatment and antibiotic conservation efforts, it is difficult to increase the attractiveness of antibiotics marketing solely by modifying price levels. Therefore, approaches towards increasing predictability of sales volumes or delinking them from revenue merit consideration.
Increasing the target population through joint purchasing options at the EU level could increase commercial viability of supplying essential generic antibiotics
At the EU level, one option for increasing the commercial viability of existing antibiotics, particularly those that are vulnerable to shortages, would be to increase target populations by exploring joint purchasing options. Given the nature of antibiotic resistance pathogens, antibiotics arguably fall within the scope of the EU Joint Procurement Agreement (JPA) (McEvoy & Ferri, 2020), which is applicable for medical countermeasures to address serious cross-border threats to health (see Box 8). In theory, cross-country joint procurement can enhance transparency through better information-sharing; enable cross-country learning by sharing experience; strengthen bargaining power and mitigate overly high transaction costs by pooling skills, capacities and negotiations; ensure sustainable access to health technologies by sharing resources through cross-border exchange of products in short supply; achieve sustainable prices through economies of scale; thereby also improving the quality of purchased goods, ensuring supply security and availability, and fostering certain innovations (Espín et al., 2016). In practice, its implementation can be resource-intensive and requires political will and flexibility for necessary trade-offs (Espín et al., 2016; European Commission et al., 2022). What is more, to maximize gains in the context of antibiotics, a priority list of products to be purchased jointly would need to be developed and agreed among participating countries (see also next section); such a prioritization would also prevent overcentralization, which could mean fewer opportunities for local marketing authorization holders to win central tenders, thus endangering their sustainability.
Box 8
Experience with joint purchasing in the EU includes the Joint Procurement Agreement and advance purchase agreements for COVID-19 vaccines.
Good procurement practice is crucial at the national level and also for any joint purchasing initiatives
Importantly, any joint purchasing efforts at the EU level should mirror good practice in national level procurement. This entails refraining from race-to-the-bottom tendering by ensuring more than one supplier is contracted, and that contracts are of sufficient duration and entail sufficient minimum order amounts to attract adequate bids and improve predictability. When it comes to the expectations of purchasers, delivery security should be considered in the procurement process, either as a criterion for awarding the contract or as a bonus reimbursement component when related targets have been achieved. Examples of such practices are already available in Europe, as showcased in a recent study exploring public procurement practice in European countries (European Commission et al., 2022). While the study focused on practices in different countries, its findings are transferrable to collaborative efforts, and cross-country collaboration is recognized as a fundamental pillar for future efforts. The exchange of good procurement practice across countries can be facilitated through existing initiatives, such as the Network of Competent Authorities on Pricing & Reimbursement (NCAPR), an informal cooperation platform steered by the European Commission. Specifically for antibiotics, the introduction of environmental criteria into procurement terms also merits consideration under the One Health approach; examples from the Nordics are already available (European Commission et al., 2022).
Delinked payment models, such as subscription payments, are promising for existing antibiotics as well
Beyond optimizing procurement practice, partially or fully delinking revenue from sales volume and price is an option that can be considered for existing antibiotics as well. The use of subscription-style payment models can be applied here too, including the mechanism for a partially delinked subscription model at the EU level proposed by JAMRAI (Box 6). The exact size of the guarantee would require discussion, as would the exact implementation of such a plan and its alignment with public health needs. Political will to agree and consider the different abilities to pay among EU Member States would be crucial for the initiation and success of such models. Sweden’s annual revenue guarantee pilot, described in section 3, partially delinks revenue from sales and includes the older antibiotic fosfomycin in addition to new products. The pilot was evaluated positively in January 2023; this should encourage additional discussions on the potential of similar models for ensuring access to existing antibiotics and addressing market failures.
A better understanding of the antibiotics supply is necessary and can pave the way for strengthening the supply chain and addressing shortages
As mentioned in section 3, antibiotics shortages typically occur because there are limited actors involved in the production and supply chain, and demand has not been possible to forecast and anticipate. Additionally, despite the overall lack of transparency around where raw materials are procured and the producers of reaction intermediaries or APIs are located, it is clear that these activities largely take place outside the EU. The next paragraphs consider policy options to improve the sustained supply of essential antibiotics through strengthening intelligence, manufacturing and stockpiling.
Mapping European pharmaceutical production capacity would facilitate prompt identification of alternative producers when shortages occur
Despite European and national obligations for market authorization holders to notify competent authorities of any foreseeable temporary or permanent disruptions in supply, a lack of transparency persists (not least due to suboptimal information-sharing among the numerous stakeholders that make up the pharmaceutical supply chain) (see Årdal et al., 2021 and Article 23a of 2001/83/EC on the European regulatory framework (European Commission, 2001; Årdal et al., 2021). A first step to counteract this problem could entail a European pharmaceutical production capacity mapping database of all industrial entities that make up the pharmaceutical supply chain. Such a mapping would allow the prompt identification of alternative producers when shortages occur and spread risks at procurement. A recent feasibility study commissioned by HERA and carried out by McKinsey identified potential measures that could help increase transparency of information in the event of shortages, as supply-chain set-up information is often protected and difficult to obtain (European Commission, 2022b, 2022d). A Staff Working Document from the European Commission also highlights the necessity for increased transparency along the supply chain, starting with raw materials prior to the registered starting material phase (European Commission, 2022d). For instance, the EU may choose to adapt the policy implemented by New Zealand, where the New Zealand Medicines and Medical Devices Safety Authority provides publicly available information on the names and locations of API producers, finished product manufacturers, product sponsors and the marketers of products (MedSafe, 2022).
An EU-level facilitated exchange between Member States could help identify and compare which antibiotic formulations are available in different settings
Another approach towards strengthening intelligence and providing a solid foundation for action to improve the supply chain would entail EU-level facilitated exchange between Member States to identify and compare which antibiotic formulations are available in different settings, and which are the best options for existing medical needs according to best available evidence. A good example of how different strengths and formulations exist without clinical necessity is the widespread use of both amoxicillin 125mg/5ml and 135mg/5ml oral suspensions in Europe. If formulations were aligned, this could help concentrate manufacturing efforts to those products that would provide the most benefit across the EU. Such a documentation initiative of available formulations in different countries could also provide the basis for a platform that could support swift action in cases of shortages in individual countries, which could use it to identify where to look for support (see a best-practice example in this direction in Box 8). Alongside aligning formulations, there are potential opportunities to harmonize the packaging and labelling of certain antibiotics to facilitate joint procurement efforts that are applicable to multiple Member States and to ensure that stockpiles of antibiotics can be deployed across Europe without the need for repackaging.
Strengthening manufacturing capacities within the EU, as well as outside China and India, could help improve availability of antibiotics
Building on this, strengthening manufacturing capacities within the EU, as well as outside China and India (see also next chapter on the EU’s engagement at the global level), could be considered. This could include subsidies to manufacturers investing in facilities within the EU, but would require careful consideration of the nature of these subsidies and the implications for competition in the common market as well the limitations imposed by rules on state aid. Given the significant infrastructure and investment requirements for developing manufacturing capacity for antibiotics, such an option merits consideration at the EU level, in addition to (and ideally in conjunction with) national initiatives. In the Pharmaceutical Strategy for Europe, the European Commission committed to “promote investment and coordinate research, development, manufacturing, deployment and use for novel antibiotics as part of the new EU Health Emergency Response Authority” (European Commission, 2020); however, such coordinated action is also necessary for existing antibiotics. A recent example in this direction is the investment of the Austrian government and Sandoz in penicillin manufacturing capacities (Wallace, 2020). It is evident that EU action aimed at strengthening manufacturing capacity is a long-term solution to supply considerations and would require substantial investment, meticulous planning and consideration of externalities.
Virtual stockpiling could help reduce shortages that are caused by supply disruptions
Relatedly, the aforementioned McKinsey analysis on addressing shortages concluded that virtual stockpiling (see Table 9), whereby an EU overview of decentralized buffers of supplies is maintained, might be a solution for shortages that are caused by supply disruptions. Combined with mandatory inventories in the private sector, such a system could also provide the basis for balancing physical stockpiles across EU Member States and determining the need for EU-coordinated procurement as described above. This would likely need to be complemented with public sector inventories for some high-risk infections, such as extensively resistant TB, where there would be benefits in having a supply of antibiotics that could be rapidly deployed to Member States. How such a virtual stockpile might be operationalized at the EU level would require additional thought, balancing benefits with the challenges of regulatory and legal change. During the COVID-19 pandemic, the European Commission upgraded the EU Civil Protection Mechanism to create rescEU, which established a new European reserve of resources (the ‘rescEU reserve’), including a stockpile of medical items and field hospitals that can respond to health emergencies. rescEU could be leveraged to store antibiotics in light of AMR-related action in the Pharmaceutical Strategy for Europe.
Increased collaboration between the relevant EU bodies as well as with the WHO Expert Committee on Selection and Use of Essential Medicines could help determine priorities for concerted action
The exact set-up of the options described above, as well as which EU agencies and mechanisms are best suited to develop and implement them, would need to be considered further. Regulation (EU) 2022/123 of the European Parliament and of the Council was adopted in January 2022 (European Commission, 2022c, p. 123), and includes a reinforced role for the EMA as regards crisis preparedness and management for medicinal products and medical devices, including shortages. HERA’s mandate explicitly includes increasing the stockpiling capacity of medical countermeasures, including through the use of EU4 Health funds (Anderson, Forman & Mossialos, 2021; Forman & Mossialos, 2021). It is clear that close collaboration with the ECDC is vital for the successful alignment of actions with public health needs. Crucially, given the fragmented governance structures for antibiotic procurement, there is currently a lack of clarity within and between health systems about the distribution of responsibility to determine which shortages pose a sufficient risk for public health to warrant concerted action; this is another area that lends itself to increased dialogue at the EU level, and would correspond to the aspirations of the Pharmaceutical Strategy for Europe. Increased collaboration with the WHO Expert Committee on Selection and Use of Essential Medicines could contribute to achieving this goal. Successful examples at the national level, such as the PLATINEA initiative in Sweden (see Box 9), can serve as blueprints for considering modalities in international collaboration, and synergies with the AMR Multi-Stakeholder Partnership Platform, launched in November 2022 and hosted by the Food and Agriculture Organization (FAO) of the United Nations, would need to be explored (FAO, 2023).
Box 9
The Swedish PLATINEA initiative is a best-practice example for a collaborative platform towards optimizing access to antibiotics.
Table 10
Selected policy options for improving access to existing antibiotics.
6. The EU’s role in global efforts to ensure sustained access to antibiotics
The EU has an interest in supporting global efforts to address AMR, not only to safeguard the health of its citizens but to contribute to overall population wellbeing
The previous chapters have focused largely on options to ensure the availability of new and existing antibiotics in the European setting. However, antibiotic resistance is a global problem, and lack of access to appropriate and effective antibiotics is a threat to global health security. What is more, lack of access to antibiotics as a driver of excess mortality is particularly felt in low-income and middle-income settings, which also most acutely experience antibiotics shortages (Shafiq et al., 2021) and would experience larger drops in economic growth with increasing AMR burden (World Bank, 2017). In that respect, the EU has an interest in supporting global efforts to address AMR, not only to safeguard the health of its citizens, but to contribute to overall population wellbeing (Anderson et al., 2019).
The EU’s new Global Health Strategy recognizes AMR as one of the key challenges and commits it to intensifying efforts to address this
An exhaustive mapping of how EU action impacts global health is beyond the scope of this policy brief, however it is important to note that the EU’s Action Plan against AMR launched in 2017 explicitly foresees a One Health approach at a global level, and the current Commission is tasked with working towards a global agreement on access to and use of antibiotics. This task is reflected in the EU’s new Global Health Strategy, adopted in November 2022, which recognizes AMR as one of the key challenges to address in the coming years and commits to a range of actions towards intensifying efforts to address this, including a commitment to support the development of new antibiotics (see Box 10). This commitment is in line with the Muscat Ministerial Manifesto, agreed by the Third Global High-level Ministerial Conference on Antimicrobial Resistance (AMR) in November 2022, which calls for increased investments in innovation against antibiotic resistance (and in particular the development of a robust antibiotic pipeline) (WHO, 2022b) and with the G20 call to action following the forum’s Bali meeting, also in November 2022 (G20 Research Group, 2022).
Box 10
The new EU Global Health Strategy contains actions to strengthen a One Health approach towards containing AMR.
The EU recognizes the importance of aligning international efforts towards addressing AMR and can build on its existing relationships to act as a facilitator in the global response to AMR
Crucially, the EU’s new Global Health Strategy explicitly recognizes the importance of aligning efforts with international organizations pursuing shared goals, such as the UN’s Quadripartite (see Box 10). The EU can build on its relationships with such institutions in conjunction with its G7 and G20 membership to act as a facilitator in the global space and leverage its voice towards advancing shared objectives and ensuring synergies among existing structures, including working towards a multinational pull incentive and improving intelligence, as suggested in previous chapters. The latter could include working towards a commitment around the ‘fair’ contribution of different countries, ideally drawing on WHO expertise. This becomes particularly timely in light of the upcoming second United Nations General Assembly High-level Meeting on AMR, scheduled for 2024.
The future pandemic agreement provides a unique opportunity to make progress in tackling AMR at the global level through the inclusion of concrete provisions, including to promote the development and availability of medical countermeasures to combat AMR. Thinking in line with the options presented in this brief, additional considerations for the EU could entail supporting investment in manufacturing capacities in low- and middle-income settings to address the fragility of supply also in those settings; instruments such as Global Europe or the Team Europe Initiatives could be considered to support such actions (European Commission, 2023a, 2023b). Finally, given its position in the global arena, the EU could increase its efforts in communicating, both within and outside its confines, what the costs of inaction in the face of antibiotic resistance entail and in actively supporting related national and regional efforts.
Footnotes
- 1
In the majority of this policy brief we refer to antibiotics that treat bacterial infections. Occasionally we have used the broader terms, antimicrobials or anti-infectives. These broader terms also encompass antiviral and antifungal treatments. However, the issues relating to antibiotics discussed are also relevant to antimicrobials and anti-infectives.
- 2
Bacteria develop resistance to antibiotics when they acquire a random change or mutation in their genetic structure or take up a mobile resistance genetic element from the environment that allows them to survive in the presence of antibiotics that are intended to kill them. This means they outgrow non-resistant bacteria and pass on this resistant trait to subsequent generations. This phenomenon is known as ‘selection pressure’, where a factor in the environment causes one type of organism to develop and grow in preference to another.
- 3
Sweden has a relatively smaller population than most EU Member States and has likely achieved better access to new antibiotics because of its pilot of annual revenue guarantees for new antibiotics. Please see section on pull incentive implementation for more details.
- 4
While the Joint Programming Initiative on Antibiotic Resistance (JPIAMR) is predominantly focused on EU Member States, many non-EU countries are represented and several funding grants involve academics from these countries.
- 5
The recommended statement is as follows: It is recommended that {agent name} should be used to treat patients that have limited treatment options only after consultation with a physician with appropriate experience in the management of infectious diseases (EMA, 2014).
- 7
This comparative assessment of incentive mechanisms is for illustrative purposes to stimulate debate and is heavily dependent upon the relative size of payment or length of exclusivity extension. The impact on public health factors is also very dependent upon relevant terms and conditions included.
- 8
All incentive mechanisms can be combined with prospective payment agreements, such as subscription-style payments that include conditions on antibiotic sustainability, patient access and environmental health.
- 9
While we analyse operational feasibility at the EU level, there is consensus among policy-makers that optimal deployment of push and pull incentives for antibiotics are likely to require a global solution, including engagement and implementation by at least all G20 or G7 countries.
- 10
The legislative framework required for implementation of incentives analysed in this policy brief should be the subject of future research to clarify legislative feasibility at the EU level.
- 6
Intellectual property rights are related to the patents and supplementary protection certificates (SPC) that are filed during the invention or creation of new drug molecules. In the EU, as is the case in most of the rest of the world, a patent is valid for 20 years. An SPC extends the IP protection period by up to 5 years, and is determined by: (date of the first market authorization – date of filing of corresponding patent – 5 years).
Regulatory data protection is protection of data submitted by a company in the context of a marketing authorization against use by others. In the EU, drug developers are granted 8 years of regulatory data protection after market authorization.
- Introduction
- What are the major challenges across the R&D pathway for antibiotics?
- What challenges exist to ensure sustainable supply of new and pre-existing antibiotics?
- What actions can be taken at the EU level to stimulate research, innovation and development of new antibiotics?
- How can the EU promote sustainable and timely access to pre-existing and new antibiotics once developed and approved?
- The EU’s role in global efforts to ensure sustained access to antibiotics
- Policy brief - How can the EU support sustainable innovation and access to effec...Policy brief - How can the EU support sustainable innovation and access to effective antibiotics?
Your browsing activity is empty.
Activity recording is turned off.
See more...