All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: tni.ohw@sredrokoob). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: tni.ohw@snoissimrep).
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Implementing the WHO Stop TB Strategy: A Handbook for National Tuberculosis Control Programmes. Geneva: World Health Organization; 2008.

Implementing the WHO Stop TB Strategy: A Handbook for National Tuberculosis Control Programmes.
Show details4.1. Special features of tuberculosis in children
Of the estimated 8.8 million new cases of TB that occurred globally in 2005, about 1 million (11%) were children aged under 15 years. Children with TB differ from adults in their response to the disease in ways that may have important implications for the prevention, diagnosis and treatment of TB. Furthermore, children are at increased risk of progression of primary M. tuberculosis infection to disease, and are therefore a target group for preventive treatment. Children also develop primary TB more commonly than adults. Although bacteriological confirmation of TB should be sought whenever possible, this is often not possible in young children with pulmonary TB who usually cannot produce a sputum sample.
In the absence of HIV infection, most children with TB come within WHO TB diagnostic Category III and should be treated during the initial phase of treatment with three drugs (isoniazid, rifampicin and pyrazinamide) for two months, followed by a continuation phase of treatment with two drugs (isoniazid and rifampicin) for four months. Children are particularly liable to develop TB meningitis and miliary TB and deserve special consideration (see the recommended treatment regimens in Guidance for national tuberculosis programmes on the management of tuberculosis in children).
There is an urgent need to improve the prevention, diagnosis and treatment of TB in children, by ensuring their inclusion in NTPs in line with international standards and guidelines. The International standards for tuberculosis care and WHO's guidelines on treatment of tuberculosis are relevant for patients of all ages, whether children or adults. The overall planning response to TB should include the response to TB/HIV and MDR-TB in children as well as in adults.
For NTPs to successfully and effectively prevent and manage TB in children, standardized approaches based on the best available evidence should be incorporated into existing NTP guidelines and strategies. The engagement of all who provide care to children (including paediatricians and other clinicians) is crucial. Reducing the burden of TB in children will require changing and improving many existing practices, such as those that relate to contact investigations. Updating the NTP recording and reporting system is necessary, in line with WHO recommendations. Operational research is essential to establish how NTPs can best ensure the delivery of effective prevention and care for childhood TB.
4.1.1. Policy changes
NTPs should be aware of two important policy changes that relate to recording and reporting and to the dosage of ethambutol.
Recording and reporting. NTPs should record and report two age groups for children (0–4 years and 5–14 years). This has considerable benefits: (i) it is crucial in ensuring the management of childhood TB as part of routine NTP activities; (ii) it is useful in ordering drugs, since child-friendly formulations are particularly important in children aged 0–4 years; (iii) it is important in monitoring of trends in these two distinct age groups, since children aged 0–4 years are the most vulnerable, and infection at these early ages indicates recent transmission; (iv) it will provide valuable and continuous information on market needs concerning child-friendly formulations of anti-TB drugs; and (v) it is consistent with age groupings used in the Integrated Management of Childhood Illness (IMCI).
Dose of ethambutol. When a child receives treatment with a regimen containing ethambutol, the revised recommended dose is 20 mg/kg (range 15–25 mg/kg) daily. A literature review indicates that ethambutol is safe in children of all ages at this dose. Ethambutol was previously often omitted from regimens for children, owing in part to concerns about optic neuritis.
4.2. Strategic approach to preventing and managing tuberculosis in children
The NTP's overall strategic approach to decrease the burden of childhood TB consists of two elements.
Prevention of TB. Measures to prevent TB involve screening children who are the household contacts of a TB case (usually an adult family member) to enable those children found to have TB to be treated and those children not found to have TB to receive isoniazid preventive therapy (IPT).
Management of TB. Measures to manage TB involve the routine diagnosis, treatment, and recording and reporting of TB in children as part of routine NTP activities, in line with international standards and guidelines. The diagnosis and treatment of drug-resistant TB in children are complex and should be carried out at referral centres.
Chapter 8 provides recommendations for immunization with bacille Calmette–Guérin (BCG), one of the childhood vaccines administered under the Expanded Programme on Immunization.
The NTP should collaborate with child health services in implementing the strategic approach to prevention and management of TB in children. The overall context for providing high-quality care to sick children is provided by the IMCI strategy promoted by WHO and the United Nations Children's Fund (UNICEF) (see Chapter 23, section 23.2).
4.2.1. Prevention of tuberculosis in children
NTPs should organize a system for screening the children of household contacts of infectious pulmonary TB cases. This enables those children found to have TB to be registered and treated, and for those children not found to have TB but who are at high risk of TB (children aged less than 5 years and all HIV-infected children) to receive IPT (i.e. daily isoniazid for at least six months).
The tuberculin skin test (TST) is the best way to detect M. tuberculosis infection, and chest X-ray (CXR) is the best method to screen for TB disease among contacts. These tests should be used where available to screen exposed contacts. However, tuberculin is often unavailable in low-resource settings. TST and CXR, when not readily available, should not preclude contact screening and management, as this can be conducted on the basis of simple clinical assessment (Figure 4.1).
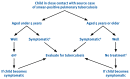
FIGURE 4.1
APPROACH TO CONTACT MANAGEMENT WHEN CHEST X-RAY AND TUBERCULIN SKIN TEST ARE NOT READILY AVAILABLEa. a Adapted from Guidance for national tuberculosis programmes on the management of tuberculosis in children Geneva World Health Organization2006(WHO/HTM/TB/2006.371). (more...)
Special situations
- Close contacts of MDR-TB patientsChildren who are close contacts of MDR-TB patients should receive careful clinical follow-up for a period of at least two years. If active disease develops, prompt initiation of treatment with a regimen designed to treat MDR-TB is recommended. WHO does not recommend second-line drugs for chemoprophylaxis in MDR-TB contacts.
- Breastfeeding infantsInfants who are being breastfed have a high risk of infection from mothers with smear-positive pulmonary TB and of developing TB. Infants should receive six months of IPT, followed by BCG immunization. Breastfeeding may be safely continued during this period.
4.2.2. Diagnosis of tuberculosis in children
The diagnosis of TB in children relies on a careful and thorough assessment of all the evidence derived from an accurate history, clinical examination and relevant investigations, e.g. TST, CXR and sputum smear microscopy. Although bacteriological confirmation of TB is not always feasible, it should be sought whenever possible, e.g. by sputum smear microscopy for children with suspected pulmonary TB who are old enough to produce a sputum sample. A trial of treatment with anti-TB medication is not recommended as a method for diagnosing TB in children. The decision to treat a child should be carefully considered; once such a decision is made, the child should be treated with a full course of therapy. Standard international case definitions apply to adults and children. In most immunocompetent children, TB presents with symptoms of a chronic disease after they have been in contact with an infectious source case. Box 4.1 outlines the key risk factors for TB in children.
BOX 4.1
KEY RISK FACTORS FOR TUBERCULOSIS. Household contact of a newly diagnosed smear-positive case Aged less than 5 years
Box 4.2 shows the key features suggestive of TB in children. Infection with M. tuberculosis can usually be demonstrated by a TST (although less reliably in the presence of HIV infection). The clinical presentation in infants may be more acute, resembling acute severe pneumonia, and should be suspected when there is a poor response to antibiotics. In such situations, there is often an identifiable source case, usually the mother.
BOX 4.2
KEY FEATURES SUGGESTIVE OF TUBERCULOSIS. A diagnosis of tuberculosis is strongly suggested in the presence of three or more of the following: chronic symptoms suggestive of tuberculosis
Existing diagnostic tests for TB in children have shortcomings, and the full range of tests (including bacteriological culture and TST) is often not available in settings where the disease is diagnosed in the vast majority of cases.
In some countries, score charts are used for the diagnosis of TB in children. These charts have rarely been evaluated or validated against a “gold standard” and should therefore be used as screening tools and not as the means of making a firm diagnosis. Score charts perform particularly poorly in children suspected of pulmonary TB and in children who are also HIV-infected.
Box 4.3 summarizes WHO'S recommended approach for the diagnosis of TB in children.
Box 4.3
RECOMMENDED APPROACH FOR DIAGNOSIS OF TUBERCULOSIS IN CHILDREN. Careful history (including history of TB contact and symptoms consistent with TB) Clinical examination (including growth assessment)
Children are equally susceptible to drug-resistant and to drug-susceptible TB. Drug-resistant TB is a laboratory diagnosis. However, drug-resistant TB should be suspected if any of the features below are present.
- Features in the source case suggestive of drug-resistant TB
- contact with a known case of drug-resistant TB;
- remains sputum smear-positive after three months of treatment;
- history of previously treated TB;
- history of treatment interruption.
- Features of a child suspected of having drug-resistant TB
- contact with a known case of drug-resistant TB;
- not responding to the anti-TB regimen;
- recurrence of TB after adherence to treatment.
4.2.3. Treatment of tuberculosis in children
Chapter 2 details the drug doses recommended by WHO for TB in children.
Children usually have paucibacillary pulmonary disease (low organism numbers), as cavitating disease is relatively rare (about 6% of cases or fewer) in those aged under 13 years (the majority of the organisms in adult-type disease are found in the cavities). In contrast, extrapulmonary TB occurs more commonly in children than in adults. Severe and disseminated TB (e.g. TB meningitis and miliary TB) occur especially in young children (aged under 3 years). Both the bacillary load and the type of disease may influence the effectiveness of anti-TB regimens. Treatment outcomes in children are generally good, even in young and immunocompromised children who are at higher risk of disease progression and disseminated disease, provided that treatment starts promptly. There is a low risk of adverse events associated with use of the recommended regimens.
The recommended anti-TB regimens for each diagnostic category are generally the same for children as for adults (see Table 2.2). New cases fall within Category I (new smear-positive pulmonary TB; new smear-negative pulmonary TB with extensive parenchymal involvement; severe forms of extrapulmonary TB; severe concomitant HIV disease) or Category III (new smear-negative pulmonary TB other than in Category I; less severe forms of extrapulmonary TB).
Most children with TB have uncomplicated (smear-negative) pulmonary or intrathoracic TB or non-severe forms of extrapulmonary TB. They therefore come within WHO TB diagnostic Category III: the recommended treatment regimen is 2HRZ/4HR (or 2HRZ/6HE). A minority of children have smear-positive pulmonary TB, extensive pulmonary involvement or severe forms of extrapulmonary TB (e.g. abdominal or TB of the bones or joints). They therefore come within diagnostic Category I: the recommended treatment regimen is 2HRZE/4HR (or 2HRZE/6HE). Children with TB meningitis and miliary TB require special consideration (see WHO Guidance for national tuberculosis programmes on the management of tuberculosis in children). Previously treated cases fall under diagnostic Category II (previously treated smear-positive pulmonary TB) or Category IV (chronic cases and MDR-TB).
Use of corticosteroids. Corticosteroids may be used for the management of some complicated forms of TB, e.g. TB meningitis, complications of airway obstruction by TB lymph glands and pericardial TB. In cases of advanced TB meningitis, corticosteroids have been shown to improve survival and decrease morbidity. Corticosteroids may be useful in some cases of immune reconstitution.
Treatment support. Children, their parents and other family members, and other caregivers should be educated about TB and the importance of completing treatment. Treatment is usually administered by the child's mother or other caregiver. Support from the child's parents and immediate family is vital to ensure a satisfactory treatment outcome. Often, a health-care worker can observe and record the treatment, but if this arrangement is not convenient for the family, a trained community member (preferably someone other than the child's parent or immediate family) can undertake this responsibility. All children should receive treatment free of charge, whether the child is smear-positive at diagnosis or not. FDCs should be used whenever possible to improve simplicity of and adherence to treatment. Patient treatment cards are recommended for documenting treatment adherence.
Hospital care. Children with severe forms of TB should be hospitalized for intensive management where possible. Conditions that merit hospitalization include: (i) TB meningitis and miliary TB, preferably for at least the first two months; (ii) respiratory distress; (iii) spinal TB; and (iv) severe adverse events, such as clinical signs of hepatotoxicity (e.g. jaundice). If it is not possible to ensure good adherence and treatment outcome on an outpatient basis, some children may require hospitalization for social or logistic reasons.
HIV-infected children. Most current international guidelines recommend that TB in children infected with HIV should be treated with a six-month regimen, as for children who are not infected with HIV. Where possible, HIV-infected children should be treated with rifampicin for the entire duration of treatment, as higher relapse rates among HIV-infected adults have been found when ethambutol is used in the continuation phase. Most children with TB, including those who are HIV-infected, have a good response to the six-month regimen. Possible causes of failure, such as non-compliance with therapy, poor drug absorption, drug resistance and alternative diagnoses, should be investigated in children who are not improving on anti-TB treatment.
All children with TB and HIV coinfection should be evaluated to determine whether ART is indicated during the course of treatment for TB. Appropriate arrangements for access to antiretroviral drugs should be made for patients who meet indications for treatment. Given the complexity of co-administration of anti-TB treatment and ART, consultation with an expert in this area is recommended before initiation of concurrent treatment for TB and HIV infection, regardless of which disease appeared first. However, initiation of treatment for TB should not be delayed. Children with TB and HIV coinfection should also receive co-trimoxazole as prophylaxis for other infections.
In HIV-infected children with confirmed or presumptive TB disease, initiation of anti-TB treatment is the priority; however, the optimal timing for ART initiation is not known. The decision on when to start ART after starting anti-TB treatment involves a balance between the child's age, pill burden, potential drug interactions, overlapping toxicities and possible IRIS versus the risk of further progression of immune suppression with its associated increase in mortality and morbidity. Many clinicians start ART 2–8 weeks after starting anti-TB treatment.
4.2.4. Recording and reporting
Children with TB should always be included in the routine NTP recording and reporting system (see Chapter 3). It is crucial to notify the NTP of all identified TB cases in children, register them for treatment and record their treatment outcome. At the end of the treatment course for each child with TB, the district TB officer should record the standard outcome in the district TB register. Four of the standard outcomes are applicable to children with smear-negative pulmonary or extrapulmonary TB: treatment completion, default, death and transfer out.
Recording and reporting two age groups for children (0–4 years and 5–14 years) in the TB registers is useful for drug procurement (in child-friendly formulations for young children) and to monitor trends of case-finding and treatment outcomes. Evaluation of treatment outcome by cohort analysis in children is a valuable indicator of the quality of programmes for child TB patients. For TB/HIV indicators in children, see Chapter 13.
4.3. Main activities to be carried out by the national TB control programme for implementation of interventions to prevent and manage tuberculosis in children
Implementation by the NTP of interventions to prevent and manage childhood TB requires the activities listed below.
- Preparations:
- advocate to health authorities for mainstreaming of “childhood TB interventions” as part of routine NTP activities, in collaboration with the maternal and child health programme;
- conduct a situation analysis of the extent to which childhood TB interventions are mainstreamed as part of routine NTP activities, of currently available data on prevention, case finding and treatment outcome, and of resources available for implementation of childhood TB interventions;
- adapt NTP guidelines to reflect childhood TB interventions;
- adapt maternal and child health guidelines to reflect childhood TB policies;
- engage with key community stakeholders (academics, activists, etc.) to develop appropriate information, education and communication (IEC) material;
- in settings with high HIV prevalence, ensure effective linkages with HIV control services.
- Facilitate meetings with relevant health and other authorities at national, regional or provincial and district level, to ensure engagement of all health providers (government, nongovernmental organizations, private sector, religious and charity organizations, etc.)
- Training to implement childhood TB interventions:
- develop and produce training material for health staff based on national guidelines;
- develop and produce training material for community participants (e.g. those involved in contact tracing and case-finding, and in promoting and encouraging adherence as “treatment supporters”);
- sensitize health staff and relevant community members regarding the nature and extent of the problem of childhood TB, and motivate them to share responsibility for contact tracing, case-finding and treatment support.
- Delivery of childhood TB interventions as part of routine NTP activities
- assess drug procurement system for effectiveness in ensuring availability of quality-assured formulations of anti-TB drugs for children (and consider obtaining these drugs through the GDF);
- monitor results of contact tracing, case-finding and treatment support;
- check how effectively the routine NTP recording and reporting system is capturing the data on case-finding and treatment outcomes;
- evaluate how effectively the full range of health providers is mobilized to contribute to making high-quality childhood TB interventions universally accessible;
- assess effectiveness of the system of procurement of tuberculin.
- Advocacy, communications and social mobilization (ACSM) activities
- incorporate messages about childhood TB in ACSM activities for health promotion;
- advocate for commitment and funds to ensure universal access to high-quality childhood TB interventions as part of routine NTP activities.
WHO guidelines
- Guidance for national tuberculosis programmes on the management of tuberculosis in children. Geneva: World Health Organization; 2006. (WHO/HTM/TB/2006.371; WHO/FCH/CAH/2006.7) [PubMed: 17044200]
- Guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: World Health Organization; 2006. (WHO/HTM/TB/2006.361)
- Treatment of tuberculosis: guidelines for national programmes. 3rd ed. Geneva: World Health Organization; 2003. (WHO/CDS/TB/2003.313)
Key references
- Antiretroviral therapy of HIV infection in infants and children: towards universal access. Recommendations for a public health approach. Geneva: World Health Organization; 2006. [PubMed: 23741772]
- Ethambutol efficacy and toxicity: literature review and recommendations for daily and intermittent dosage in children. Geneva: World Health Organization; 2006. (WHO/HTM/STB/2006.365)
- Improving the management of childhood tuberculosis within national tuberculosis programmes: research priorities based on a literature review. Geneva: World Health Organization; 2007. (WHO/HTM/TB/2007.381)
- International standards for tuberculosis care. The Hague: Tuberculosis Coalition for Technical Assistance; 2006.
- Marais BJ, et al. Childhood pulmonary tuberculosis. Old wisdom and new challenges. American Journal of Respiratory and Critical Care Medicine. 2006;174:1078–1090. [PubMed: 16484674]
- Marais BJ, et al. Diversity of disease manifestations in childhood pulmonary tuberculosis. Annals of Tropical Paediatrics. 2005;25:79–86. [PubMed: 15949195]
- Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. International Journal of Tuberculosis and Lung Disease. 2004;8:636–647. [PubMed: 15137548]
Official statement. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Chapters 1–5 in the series:
- –Chapter 1. Introduction and diagnosis of tuberculosis in children. International Journal of Tuberculosis and Lung Disease. 2006;10(10):1091–1097. [Google Scholar]
- –Chapter 2. Anti-tuberculosis treatment in children. International Journal of Tuberculosis and Lung Disease. 2006;10(11):1205–1211. [Google Scholar]
- –Chapter 3. Management of TB in the HIV-infected child. International Journal of Tuberculosis and Lung Disease. 2006;10(12):1331–1336. [Google Scholar]
- –Chapter 4. Childhood contact screening and management. International Journal of Tuberculosis and Lung Disease. 2007;11(1):12–15. [Google Scholar]
- –Chapter 5. Health staff roles and responsibilities, recording and reporting and BCG vaccination. International Journal of Tuberculosis and Lung Disease. 2007;11(2):134–137. [Google Scholar]
- Weismuller MM, et al. Diagnosis of childhood tuberculosis in Malawi: an audit of hospital practice. International Journal of Tuberculosis and Lung Disease. 2002;6:432–438. [PubMed: 12019919]
- PubMedLinks to PubMed
- Tuberculosis in children - Implementing the WHO Stop TB StrategyTuberculosis in children - Implementing the WHO Stop TB Strategy
Your browsing activity is empty.
Activity recording is turned off.
See more...