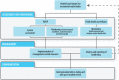
Health-based targets are measurable health, water quality or performance objectives that are established based on a judgement of safety and on risk assessments of waterborne hazards. There are two distinct types of health-based targets relevant for recreational waters:
For recreational water quality, the principal health-based targets relate to the adverse health effects associated with faecal pollution (Chapter 4) and harmful algal/cyanobacterial blooms (Chapter 5). Other hazards that may be locally or seasonally important include other microbial hazards (Chapter 6), contaminants in beach sand (Chapter 7), certain chemicals (Chapter 8), and hazards relating to aesthetics and nuisance (Chapter 9).
Details on derivation of health outcome targets for faecal–oral disease and microbial water quality targets (Recommendation 1.1) are detailed below.
2.1.1. Health outcome targets
Should a jurisdiction choose to develop health outcome–based targets, a considerable body of epidemiological information is available that may be adapted using high-quality locally relevant epidemiological studies, where available, and a national-level judgement of tolerable risk for the exposed population.
Numerous studies have shown a causal relationship between gastrointestinal symptoms and recreational water quality, as measured by levels of faecal indicator organisms (FIOs). Gastrointestinal symptoms are the most frequent health outcome for which significant dose-related associations have been reported (Wiedenmann et al., 2006). Randomized controlled trials conducted in marine waters in the United Kingdom (Kay et al., 1994; Fleisher et al., 1996) provide the most convincing data, and the most accurate measures of exposure, for water quality and illness. These trials are therefore the key studies for the derivation of guideline values for coastal and fresh recreational waters (refer to section 2.1.2). However, these results primarily apply to healthy adults using sewage-affected marine waters in temperate climates. Most studies reviewed by Prüss (1998) suggested that symptom rates were higher in younger age groups, and the United Kingdom studies may therefore systematically underestimate risks to children (Wade et al., 2008; Leonard et al., 2018).
Epidemiological studies are preferred as the basis for setting health outcome–based targets since they can eliminate sources of bias and error in assessment of human health impact. However, epidemiological studies are limited to a single, or a few closely related, diseases and carefully defined cohorts, and hence generally do not measure the full range of variation in population responses or environmental scenarios. Most recreational bathing studies have focused on temperate, not tropical, water environments, and the relationships between FIOs and pathogen survival may differ between these two environments (Harwood et al., 2014; Wade et al., 2018).
In resource-constrained settings, epidemiological studies may be challenging. Quantitative microbial risk assessment (QMRA) can be used to indirectly estimate the risk to human health by predicting infection or illness rates, given densities of particular pathogens in recreational waters, assumed rates of ingestion and appropriate dose–response models for the exposed population. QMRA estimates and epidemiological investigations have given comparable results for potential impacts of such events (Viau, Lee & Boehm, 2011; Soller et al., 2017), giving credence to the use of QMRA. QMRA can also explore risks below epidemiologically detectable levels or under circumstances that are not suited to epidemiological examination. However, caution is required in interpreting the results of QMRA because the risk of infection or illness from exposure to pathogenic microorganisms is subject to many uncertainties. Consequently, QMRA has greatest utility in resource-constrained settings for risk management (refer to section 4.4), where relative changes in estimated risks under various scenarios can be explored.
In the absence of high-quality, locally relevant epidemiological studies, national authorities are advised to develop microbial water quality targets derived from Kay et al. (1994) and Fleisher et al. (1996), as summarized in section 2.1.2 and .
2.1.2. Water quality standards
Guidance on setting national microbial water quality standards for the primary risk of faecal pollution (Recommendation 1.1) is detailed below. Indicators and guideline values for harmful algal blooms (Chapter 5), beach sand (Chapter 7) and chemical risks (Chapter 8) are included in each of the supporting chapters and summarized in the executive summary. For microbial hazards with insufficient information to develop specific guideline values (Chapter 6), operational monitoring options can be used in the context of an RWSP. Guideline values for aesthetic and nuisance aspects are presented in qualitative rather than quantitative terms since they reflect societal and cultural norms. Similarly, the quality of water that has special religious significance is also not quantified.
The guideline values presented are not mandatory limits; rather, they are measures of the safety of a recreational water environment. Derivation of guideline values and their conversion to national standards therefore require an element of valuation to address the frequency, nature and severity of associated health effects, since there is no clear cut-off value at which health effects are excluded. Societal norms play an important role in this valuation process, and the conversion of guidelines into national policy, legislation and standards should therefore take account of environmental, social, cultural and economic factors.
The existence of a guideline value or national standard does not imply that environmental quality should be allowed to degrade to this level. Indeed, a continuous effort should be made to ensure that recreational water environments are of the highest attainable quality and managed in a proactive manner. Many of the hazards associated with recreational use of the water environment are relatively short term. Short-term deviations above guideline values or conditions are therefore important to health, and measures should be in place to ensure and demonstrate that recreational water environments are continuously safe during periods of actual or potential use.
When a guideline value is exceeded, this should be a signal to:
investigate the cause of the failure and the likelihood of future failure;
liaise with the authority responsible for public health to determine whether immediate action should be taken to reduce exposure to the hazard; and
determine whether measures should be put in place to prevent or reduce exposure under similar conditions in the future (refer to
Chapter 3).
Predictive models, coupled with timely public communications, can prevent exposure by alerting water users in real time to likely exceedances (refer to section 4.2.3).
Guideline values for microbial water quality
Quantitative epidemiological studies (Kay et al., 1994) in marine water enable estimation of the degree of health protection (or, conversely, the burden of disease) associated with a range of water quality criteria. Derived guideline values for both marine and fresh water were first presented in the World Health Organization (WHO) Guidelines for safe recreational water environments (WHO, 2003), based on a tolerable burden of <1–5% gastrointestinal disease for voluntary recreational activities.
A subsequent study in fresh water (Wiedenmann et al., 2006) was used as a basis for slightly less stringent guideline values for fresh water in the later 2006 European Union Bathing Water Directive (EU, 2006), in which marine standards are generally applied to brackish or estuarine waters. Kay et al. (1994) found that enterococci best predicted gastrointestinal illness in recreational water users, whereas Wiedenmann et al. (2006) suggested that no-observed-adverse-effect levels, with respect to gastroenteritis, were evident for Escherichia coli, intestinal enterococci, somatic coliphages and Clostridium perfringens.
In these WHO Guidelines on recreational water quality: volume 1 – coastal and fresh waters, the marine water guideline values have again also been applied to fresh waters. This is based on a precautionary approach to fresh water, where effluent dilution and dispersal of untreated intermittent storm drainage is often constrained after discharges to rivers and lakes. Further, WHO recommends intestinal enterococci only, rather than intestinal enterocci and/or E. coli, since no statistical relationship has been established for E. coli that can support a dose–response guideline value. Some jurisdictions, such as the European Union, use E. coli in fresh water with a 100 cfu/100 mL threshold of risk, based on findings of Wiedenmann et al. (2006). However, the study sites in Wiedenmann et al. (2006) are less characteristic of waters globally, and use of two FIOs can introduce avoidable complexity in analysis and interpretation of results at the operational level.
As further empirical epidemiological data become available, it may be possible to use E. coli, microbial source tracking markers and viral pathogens (Gitter et al., 2020; Schoen et al., 2020) or their indicators (e.g. phages), protozoa or helminths to assess health risk in recreational waters.
The current recommended approach defines a range of water quality categories for classifying individual locations. The use of multiple categories provides incentive for progressive improvement by achieving higher water quality standards that are more protective of public health.
Coastal water
The guideline values for microbial water quality given in are derived from the key studies (Kay et al., 2004) corresponding to Recommendation 1.1. The guideline value threshold for no-observed-adverse-effect level or lowest-observed-adverse-effect level for gastrointestinal illness and acute febrile respiratory illness is 200 cfu/100 mL, corresponding to the upper range for Category B in .
Guideline values for microbial quality of coastal and freshwater recreational waters.
The values are expressed in terms of the 95th percentile – that is, the value of intestinal enterococci per 100 mL below which 95% of environmental samples would be expected to occur. They represent readily understood levels of risk based on the exposure conditions of the key studies. The values may need to be adapted to take account of different local conditions and new epidemiological studies. They are recommended for use in the classification scheme for recreational water environments discussed in section 4.3.
Fresh water
Recommended guideline values for fresh water are the same as the values for marine water in . Gastrointestinal illness occurs at a higher rate in seawater swimmers than in freshwater swimmers at a given level of faecal indicator bacteria (WHO, 2009). This difference may be due to the more rapid die-off of indicator bacteria than pathogens (especially inactivation of viruses) in seawater compared with fresh water (WHO, 2009). This would result in more pathogens in seawater than in fresh water for the same culture-derived density of FIOs.
Application of the guideline values derived for seawater to fresh water from culturable FIOs would therefore be likely to result in a lower illness rate in freshwater users, providing a conservative (i.e. more protective) guideline in the absence of suitable epidemiological data for fresh waters. However, a number of national and international authorities have different standards for seawater and freshwater sites (e.g. European Union, since 2006), based on the randomized controlled trials of Wiedenmann et al. (2006) for recreational fresh waters.
Adaptation of guideline values to national and local circumstances
The guideline values in Table 4.1 were derived from studies involving healthy adult recreational water users swimming in sewage-affected marine waters in a temperate climate. They may not apply in tropical or brackish waters, or to children, the elderly or people who are immunocompromised, who may have lower immunity and might require a greater degree of protection. If there are significant water user groups in an area, or human excreta–borne pathogen conditions differ substantially from those in temperate waters, local authorities may need to adapt the guideline values.
Risks are also likely to be greater in areas with higher carriage rates or prevalence of diseases that could be transmitted through recreational water contact, and stricter standards may be judged appropriate by local authorities if they can also be followed up with appropriate management and control actions.
If a region is an international tourist area or only used for special events, the susceptibility of visiting populations to locally endemic disease (e.g. hepatitis A) and the risk that visitors might introduce unfamiliar pathogens to the resident population need to be considered. Special events where samples have been taken to make decisions are further discussed in section 4.3.3.
Because pathogens and FIOs are inactivated at different rates, any one FIO is, at best, only an approximate index of the efficacy of pathogen removal in water (Davies-Colley, Donnison & Speed, 2000; Sinton et al., 2002; Maraccini et al., 2016; Boehm, Graham & Jennings, 2018; Jennings et al., 2018; Nelson et al., 2018; Box 4.3). This suggests that factors influencing FIO die-off should be taken into consideration when applying the guideline values in Table 4.1, depending on local circumstances. This is particularly the case where sewage is disinfected before release because disinfection may markedly increase the pathogen to indicator ratio, as described by QMRA studies (Schoen, Soller & Ashbolt, 2011).