NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 4: Antibiotic Prescribing Behavior). Rockville (MD): Agency for Healthcare Research and Quality (US); 2006 Jan. (Technical Reviews, No. 9.4.)

Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 4: Antibiotic Prescribing Behavior).
Show detailsRationale for Reducing Excess Antibiotic Use in Ambulatory Practice
The introduction of antibiotic therapy into medical practice has yielded substantial benefits for patients over the past 6 decades. Recently, however, there has been a growing awareness that the benefits of antibiotic use to individual patients come at a societal cost: the emergence of antimicrobial resistance (AMR) among bacterial pathogens. 3, 4 Though initially restricted to hospital settings, antibiotic resistance is now growing among important community-acquired bacterial pathogens, particularly Streptococcus pneumoniae (SP), 5 – 7 Staphylococcus aureus, 8, 9 and Escherichia coli . 10 – 12
Although the problem of antibiotic resistance can be viewed from many perspectives, a pragmatic viewpoint approaches it as a problem of antibiotic prescribing practices. Antibiotic use promotes the development and spread of antibiotic-resistant bacteria by at least two mechanisms: (1) selective pressure that enhances the proliferation and sustainability of bacterial strains that contain mutations conferring resistance to a specific antibiotic (or class of antibiotics), and (2) elimination of an individual's normal flora, thus conferring a survival advantage to antibiotic-resistant bacteria should exposure to antibiotics occur (i.e., creating a susceptible host). While development of antibiotic resistance primarily occurs via the first mechanism, the rapid rise in prevalence of antibiotic-resistant bacteria is primarily a function of the second mechanism. Carriage, transmission, and infection with antibiotic-resistant bacteria are strongly associated with prior antibiotic use. 13 – 16
Community-acquired antibiotic-resistant infections affect morbidity, mortality, and health care costs. Treatment failures have been reported in patients with pneumococcal meningitis after treatment with penicillin, chloramphenicol, clarithromycin, ceftriaxone, and cefotaxime. 17 – 22 The management of children with recurrent or persistent otitis media or sinusitis has become significantly more difficult as a result of the emergence of high rates of drug-resistant Streptococcus pneumoniae (DRSP) in this subgroup. 23 – 25 One US study reported an association between high-level DRSP and excess mortality among patients surviving more than 4 days in the hospital, after controlling for age and comorbidity, 26 and another demonstrated an increased risk of suppurative complications for patients with bacteremic pneumonia due to DRSP. 27
The emergence of drug-resistant bacteria has implications not only for patients with documented resistance, but for all patients who might have such infections. Currently, the specter of DRSP and other resistant bacteria has led to recommended empiric treatment regimens for a variety of infections (such as community-acquired pneumonia 28, 29 ) that include antibiotics previously reserved for life-threatening infections; antibiotic resistance to these powerful agents—often our last lines of defense—will certainly accelerate as a result. Only 6 years after the introduction of extended-spectrum macrolides, macrolide resistance among S. pneumoniae increased from 10% to 20%. 30 In Canada, where ambulatory use of levofloxacin (the agent of choice for treatment of DRSP infections) has been very high, investigators have already detected the emergence of levofloxacin resistance. 7 Previous optimistic predictions about “the end of the era of infectious diseases” have increasingly been replaced by dire predictions of a new “post-antibiotic era.” 3, 31, 32
Reducing inappropriate use of antibiotics is a critical step in preventing or slowing the progression of resistance. Progress will require a two-part approach. First, it is critical to reduce the use of antibiotics in conditions for which these drugs provide little or no benefit. Second, it is important to modify the choice of drug given to patients who do require antimicrobial therapy, and in some cases to shorten inappropriately lengthy treatment courses. Based on the best evidence and mathematical modeling to date, reductions in antibiotic consumption may not lead to major reductions in existing levels of antibiotic resistance among community-acquired bacterial infections, 33 – 35 although studies have demonstrated reduced levels of resistance to specific antibiotics. 36 However, ecological studies do support the notion that the amount of antibiotic consumption in a community directly influences how rapidly new resistance emerges or rises. 7, 37
Current Antibiotic Prescribing Practice Patterns in US Ambulatory Practices
In the US, the majority of outpatient antibiotic prescriptions are for acute respiratory tract infections (ARIs) 2 ; in 1998, an estimated 76 million ambulatory office visits for ARIs resulted in 41 million antibiotic prescriptions. 38 Based on a comparison of bacterial prevalence estimates to prescribing rates, it appears that 55% of total antibiotics prescribed for ARIs in 1998 (n=22.6 million prescriptions) were unlikely to be treating a bacterial infection, resulting in a total cost for excess antibiotic prescriptions of approximately $726 million.
In response to efforts to publicize overuse of antibiotics, 39, 40 initial progress to reduce inappropriate antibiotic prescribing was made. Between 1990 and 2000, antibiotic prescription rates for adults and children with ARIs declined significantly. Unfortunately, prescribing rates stabilized in 1999-2000. 40
In addition to the general inappropriate use of antibiotics in conditions not requiring antimicrobial therapy, increasing attention has been paid to the choice of antibiotic for patients receiving such therapy. The use of broad-spectrum antibiotics (quinolones, second- and third-generation cephalosporins, newer-generation macrolides, and amoxicillin-clavulanate) in community-based settings rose from 24% of total adult antibiotic prescriptions in 1991-1992 to 48% 7 years later. 41 A similar rise (23% to 40%) was observed in children.
A variety of national and international organizations have recommended action to improve antibiotic treatment and selection 3 . In 1996, a task force convened by the Centers for Disease Control and Prevention (CDC) outlined strategies for addressing this epidemic, highlighting the importance of promoting judicious antibiotic use in ambulatory practice. 42 The issue was further underscored by a subsequent Institute of Medicine (IOM) report on antimicrobial resistance, 43 as well as in the recommendations from a US federal multi-agency task force that created the “Public Health Action Plan to Combat Antimicrobial Resistance.” 44 In collaboration with the CDC, the National Committee for Quality Assurance and the Council for Affordable Quality Healthcare are creating quality measures for appropriate antibiotic use for inclusion in the Health Plan Employer Data and Information Set (HEDIS) 45 . Other countries have also developed their own campaigns, some with striking success. 36 Almost 30 countries in Europe have combined their efforts to monitor trends in antibiotic use. 46 Finally, in the seminal IOM report “Priority Areas for National Action: Transforming Health Care Quality,” 47 antibiotic overuse (as a major part of medication management) was identified as one of 20 priority areas for improving health care quality — a designation reflecting not only the severity of the problem, but also the substantial potential to narrow the gap between actual and best practices.
The Purpose of This Evidence-based Practice Center Report
In this report, we systematically review published research articles evaluating interventions to improve antibiotic prescribing practices. Our intent is to identify the most effective strategies for improving the use and selection of antibiotics. We focus our review on acute infectious illnesses in the outpatient setting, and separately review (1) the antibiotic treatment decision (i.e., the use of antibiotics for conditions generally not requiring antibacterial therapy), and (2) the antibiotic selection decision (i.e., the choice of one antibiotic over another, for conditions requiring antibacterial therapy). In particular, our research questions are:
- 1.
Which QI strategies are most effective in reducing unnecessary antibiotic treatment of acute illnesses?
- A.
Are particular QI strategies more effective for certain target conditions?
- B.
Are particular QI strategies more effective in specific patient populations?
- C.
Do QI strategies to reduce unnecessary antibiotic treatment affect clinical outcomes?
- 1.
Do they reduce adverse drug events?
- 2.
Do they improve clinical outcomes?
- 3.
Do they increase return visits or illness-related hospitalizations?
- D.
Do QI strategies to reduce unnecessary antibiotic treatment have downstream patient- and system-level consequences?
- 1.
Do they reduce antimicrobial resistance?
- 2.
Do they reduce antibiotic/pharmacy costs?
- 3.
Do they decrease patient satisfaction?
- 2.
Which QI strategies are most effective in improving antibiotic selection for the treatment of acute illnesses?
- A.
Are particular QI strategies more effective when the goal is (i) to reduce use of broad-spectrum agents, or (ii) to increase use of broad-spectrum agents (i.e., due to local resistance patterns)?
- B.
Are particular QI strategies more effective at improving optimal dosing and duration of a selected antibiotic therapy?
- C.
Are particular QI strategies more effective for certain target conditions?
- D.
Are specific QI strategies more effective in specific patient populations?
- E.
Do QI strategies to improve antibiotic selection affect clinical outcomes?
- 1.
Do they reduce adverse drug events?
- 2.
Do they improve clinical outcomes?
- 3.
Do they increase return visits or illness-related hospitalizations?
- F.
Do QI strategies to improve antibiotic selection have downstream patient- and system-level consequences?
- 1.
Do they reduce antimicrobial resistance?
- 2.
Do they reduce antibiotic/pharmacy costs?
- 3.
Do they decrease patient satisfaction?
In order to maximize the relevance of our findings for organizations that are considering implementing these interventions, we summarize the existing evidence by attempting to answer the following questions:
- 1.
Are quality improvement strategies to improve outpatient antibiotic use effective?
- 2.
What are the critical components of effective intervention strategies?
- 3.
Which patients and conditions should be targeted in order to exert the maximal impact on antibiotic prescribing?
- 4.
What are the limitations of current research in this field, and what areas require further study?
An Explanatory Model of Antibiotic Prescribing Behavior
The decision to prescribe antibiotics is the result of complex interactions among patient, physician, and system factors, and effective strategies to improve antibiotic prescribing behavior may need to address each of these domains. Previous research on the influence of these factors on office-based antibiotic prescribing is summarized below. Most of this research has focused on the antibiotic treatment decision, although several studies have explored factors that influence antibiotic selection.
Past research has identified the characteristics of successful QI programs across a variety of disease states and clinical settings. 48 – 51 While this work provides an important framework for examining QI strategies, factors that drive antibiotic prescribing decisions draw on a differently prioritized set of inputs from those that impact physician behavior in other clinical settings.
Specifically, the management of common outpatient infections and the decision to prescribe antibiotics, particularly when they are not indicated, can be viewed within a sociocultural and social ecological context in addition to the traditional biomedical perspective. The various inputs to this decision are presented in the adaptation of Kleinman and colleagues' model 52 for clinician decisionmaking in Figure 1 .
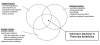
Figure
Figure 1. Factors affecting the decision to prescribe antibiotics.
Clinician Factors
Patient and parent expectations are consistently identified as the primary factor influencing unnecessary prescribing of antibiotics. 53 – 58 Nonetheless, internal clinician factors such as knowledge, experience, and training also play a role, suggesting that intervention strategies include efforts to improve clinician education.
Despite strong evidence that antibiotics are ineffective for acute bronchitis, 50 – 80% of adults with bronchitis are prescribed them. 38, 59, 60 Practice-based studies repeatedly show that there are few or no predictors of antibiotic treatment for acute bronchitis, suggesting that the diagnosis of acute bronchitis is often viewed as an indication for antibiotic treatment. Antibiotic prescribing for uncomplicated ARIs is strongly associated with the presence of purulent manifestations (purulent nasal discharge, purulent phlegm production, and tonsillar exudate). 61 These findings suggest that clinicians appear to use a heuristic (or “rule of thumb”) for deciding which patients with ARIs to treat with antibiotics, one that pays particular heed to the presence of purulence. However, purulence does not predict bacterial infection or antibiotic responsiveness among patients with ARIs. 62 – 66
Other studies have found that clinician specialty and level of training are also associated with antibiotic use for these conditions. High prescribers of antibiotics for colds, ARIs, and bronchitis are more likely to be older, 67 and to practice in rural locations. 38 In a national study of antibiotic treatment of ARIs in emergency departments, antibiotics were prescribed less often by house staff than by staff or other physicians, and more often to adults than children, regardless of specific ARI diagnosis. 68 In several national studies evaluating antibiotic selection, physician specialty was one of the strongest independent predictors of antibiotic selection, suggesting possible differences in knowledge or the “culture of prescribing.” 69 – 71
Public/Patient Factors
Multiple studies demonstrate that patients who seek care for ARIs expect to receive antibiotics, 69 – 73 and that patients or parents who expect antibiotics receive them more frequently. 54, 55, 57, 58 These expectations are strongly associated with the patient's previous experiences of receiving antibiotic treatment for these illnesses, 74 and appear to arise from misperceptions about antibiotic efficacy for viral illnesses. 75 Illness labeling may play an important role in conveying antibiotic treatment necessity. For example, referring to an acute cough illness as a “chest cold” was associated with much lower beliefs that antibiotics are necessary treatment than when the same illness was called “bronchitis.” 76
Although patients and parents frequently expect antibiotics for ARIs, most studies find that satisfaction appears more closely related to how much time the doctor spent explaining the illness rather than whether the patient received an antibiotic. 55, 57, 77, 78 In a setting where antibiotic prescribing for acute bronchitis had been reduced by 50%, patient satisfaction with care and number of return office visits did not change. 77
Patient sociodemographic factors are also associated with excess antibiotic use for ARIs, and may in part be related to varying knowledge, attitudes, and expectations in different population groups as well as clinician attitudes about these groups. Antibiotic prescription rates for ARIs appear to be lower among blacks than whites, 38, 79 which may account for the lower relative risk of carriage or infection with antibiotic-resistant bacteria among black populations. 14, 38, 79 – 81 Similarly, black patients receiving an antibiotic for ARI were less likely to be given a broad-spectrum agent than white counterparts. 69 Age also plays an important role. The frequency of antibiotic treatment for colds, ARIs, and bronchitis is greatest among the very young (age < 5 years) and lowest among the elderly (age > 64 years). 38, 60 The mechanisms by which these sociodemographic factors influence clinician decisionmaking are not known, but are probably related to varying patient expectations for antibiotics and/or clinician judgments about the ability to adequately discuss treatment choices with patients whose backgrounds differ from theirs, or who have low health literacy. 82
Health Care Delivery System Factors
Health plan and physician practice characteristics may act as barriers to, or facilitators of antibiotic prescribing for ARIs. Health plans can influence their members' propensity to seek care and expectations for care by instituting visit and pharmacy co-payments, 83 requiring prior authorization for urgent care or same-day clinician visits, and making telephone advice service available. 84 Lack of insurance also exerts an effect. 69 Health plans can influence clinician behavior by restricting formularies. Such restrictions influence both prescription antibiotic selection and patients' access to alternatives to antibiotic treatment, including non-prescription agents. 69 Practice characteristics such as location, clinician panel size, protocols for mid-level clinicians, exposure to pharmaceutical sales representatives, and availability of pharmaceutical samples also affect access and treatment decisions. For example, clinicians with greater patient workloads prescribe antibiotics for ARIs more frequently. 38, 85, 86 Lack of patient continuity and inadequate followup care mechanisms may provide additional challenges to antibiotic improvement programs based in acute care settings such as urgent care clinics and emergency departments.
Footnotes
- 2
The terms acute respiratory infection (ARI) and upper respiratory infection (URI) are frequently used interchangeably in the literature. For this report, we use ARI to refer to these conditions.
- 3
Key initiatives include the CDC's “Get Smart” campaign ( http://www
.cdc .gov/drugresistance/community/ ), the WHO's Global Strategy for Containment of Antimicrobial Resistance ( http://www .who.int/csr /resources/publications /drugresist/WHO _CDS_CSR_DRS_2001_2_EN/en/ ), and the Association for Prudent Use of Antimicrobials ( http://www .tufts.edu/med/apua/ ).
- Rationale for Reducing Excess Antibiotic Use in Ambulatory Practice
- Current Antibiotic Prescribing Practice Patterns in US Ambulatory Practices
- The Purpose of This Evidence-based Practice Center Report
- An Explanatory Model of Antibiotic Prescribing Behavior
- Clinician Factors
- Public/Patient Factors
- Health Care Delivery System Factors
- Introduction - Closing the Quality Gap: A Critical Analysis of Quality Improveme...Introduction - Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 4: Antibiotic Prescribing Behavior)
Your browsing activity is empty.
Activity recording is turned off.
See more...