This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-.

StatPearls [Internet].
Show detailsContinuing Education Activity
Endogenous endophthalmitis is a rare and dreaded ophthalmic emergency that usually results in vision-threatening sequelae. The diagnosis is often a challenge for all ophthalmologists and vitreoretinal surgeons and is generally secondary to underlying secondary systemic pathology. In a few cases, sometimes the cause may not be identified. It can manifest at any age and has varied predisposing risk factors. The etiology is multifactorial with a broad spectrum of microorganisms. Medical or surgical management depends on the manifestation and severity of the pathology. Prompt diagnosis, meticulous management, and regular follow-up are mandated for managing this serious entity. This activity describes the risk factors, etiology, clinical manifestations, investigations, and treatment protocols used by the interprofessional team.
Objectives:
- Describe the pathogenesis of endogenous endophthalmitis.
- Review the clinical features of endogenous endophthalmitis.
- Summarize the treatment of endogenous endophthalmitis.
- Outline the complications and differential diagnosis of endogenous endophthalmitis.
Introduction
Endophthalmitis, a dreaded ophthalmic emergency, is defined as the intraocular infection of the coats of the eyeball associated with diffuse vitreous inflammation. It can manifest in significant ocular and systemic complications.[1] The mode of spread can be exogenous or endogenous.[2] Exogenous endophthalmitis can result from trauma, post-surgery, or secondary to keratitis. In contrast, endogenous endophthalmitis, also called metastatic endophthalmitis, results from hematogenous spread of microorganisms secondary to underlying systemic pathology like diabetes, immunosuppression, renal pathology, etc. Suspected cases should undergo imaging like ultrasound B scan, aqueous and vitreous tap for culture and sensitivity, vitreous biopsy, or polymerase chain reaction whenever indicated for rapid diagnosis.[3]
Prompt clinical diagnosis, targeted investigation, meticulous management, and regular follow-up is mandated to safeguard vision in these cases. Medical management includes topical and systemic antibiotics or antifungals, intravitreal antibiotics or antifungals, and surgical treatment of choice is pars plana vitrectomy. The collaboration of the treating ophthalmologist or vitreoretinal surgeon, microbiologists, pathologists, and the critical care physician plays a vital role in determining the patient's final systemic and ocular outcome.[4]
Etiology
Endogenous endophthalmitis (EE) has a multifactorial etiology, and causative agents vary from region to region. The causative agents can be fungal or bacterial, with fungal organisms dominating the majority of cases.[4] In studies from Asia, fungal EE accounts for nearly 11.1 to 17.54% of total cases.[5] The most common causative agent for bacterial EE is gram-positive streptococcus and staphylococcus. Gram-negative bacterial EE is more commoner in Asian countries.[6] Among fungal species, Candida albicans account for many cases being the most common yeast, and among the molds, Aspergillus flavus is the commonest.[7]
Klebsiella pneumonia, a gram-negative rod bacterium, is another causative agent reported to cause EE. Klebsiella has been reported in patients with liver abscess, diabetes mellitus, infective endocarditis, and after a routine colonoscopy.[8] Recently, Rudez et al. reported a case of EE secondary to Clostridium septicum.[9] Yue et al. reported EE secondary to an opportunistic pathogen Citrobacter koseri.[10]
In fungal EE, mold infections are more common after chemotherapy and in patients undergoing liver and cardiac transplants, hematological malignancy, or hematopoietic stem cell transplantation (HSCT). Aspergillus pneumonia has been blamed for causing EE in a small subset of patients.[11] Candida EE has been reported in patients on ventilators and neonates.[12]
The most common systemic risk factors for EE are diabetes mellitus, immunosuppression, patients on long term corticosteroids, history of recent hospitalization, ventilator-associated pneumonia, urinary tract infection, malignancy like lymphoma and leukemia, colon adenocarcinoma, dental infection, scleral perforation, vertebral osteomyelitis, liver abscess, asplenia, hypogammaglobulinemia, cardiac or renal transplant, acquired immune deficiency syndrome, chronic alcoholism, intravenous catheters and drug abuse (Bacillus cereus). Mishra et al. reported a case of EE secondary to multifocal staphylococcal brain abscess associated with septic foci.[13][14][15][16][17][18]
Neonatal EE is an alarming entity in children with bacterial infections, candida infection, malnourishment, retinopathy of prematurity, low birth weight, septicemia. The most common bacteria reported are streptococci, S. agalactiae, Pseudomonas, and Klebsiella. Azar et al., in their retrospective analysis, reported 3 cases of pediatric EE out of 26 cases. A small number of EE cases are also reported secondary to occult systemic infections, which are culture negative.[12][19][20] Recently few case series have been reported in patients with COVID-19 possible reason being immunosuppression.[21][22][23]
Epidemiology
The reported incidence of EE is 2 to 8%.[1][4] The first case of EE dates back to 1856, which was secondary to recurrent Klebsiella infection.[24] A major review article in 2003 reported 335 cases of bacterial EE.[25] The infection can occur at any age with equal gender predilection. In a study comprising 27 subjects, the reported mean age of the patients was 63.[26] The ratio of involvement of the right to left eye is 2 to 1. The right eye is more commonly involved due to more proximal and direct blood flow from the right carotid artery. Approximately 25% of cases have a bilateral presentation.[27]
Pathophysiology
Endogenous endophthalmitis is a blood-borne metastatic infection from the primary inoculation site secondary to bacterial or a fungal pathology.[27] The microorganism primarily spreads through the posterior segment vessels. The right eye has more predilection due to dominant and direct blood flow from the right carotid artery.
The direct infectious spread has also been reported from the brain to the optic nerve. The primary mechanism of damage in EE is septic emboli that migrate to the posterior segment vessels. This, in turn, acts as a focus for the spread of microorganisms to the neighboring tissues after breaching the blood-retinal barrier. Further, the microorganism proliferates and causes inflammation within these tissues.
The infection then spread from the posterior segment to the anterior segment of the eye. One of the previous studies document no underlying source of infection in approximately 44% of the cases, but still, EE patients should undergo a detailed systemic evaluation to rule out the primary focus of infection.[28][29]
History and Physical
The symptoms of endogenous endophthalmitis vary from pain, redness, irritation, swelling photophobia, defective vision, floaters, and flashes. The clinical signs range from periorbital edema, scleral inflammation, chemosis, conjunctival congestion, corneal infiltrate, endothelial exudates, corneal edema, anterior chamber reaction, hypopyon, fibrinous membrane, iris exudates, iris nodules, sluggish or fixed pupil, relative afferent pupillary defect, posterior synechiae, cataract, absent red reflex, vitreous haze, anterior vitreous cells, vitreous floaters, membranes and exudates.[30][14][31] Bacterial EE is known to cause more anterior chamber inflammation. Bilateral EE is more common with Mycobacterium tuberculosis.
The most important diagnostic feature of EE is vitreous involvement. Aspergillus flavus is known to cause yellow/ white exudates in the vitreous, which vary from focal to diffuse. The hallmark feature of Candida EE is the presence of fluffy cotton wool-like white retinal exudates or colonies along with vitritis.[32] Subretinal and choroidal abscesses have also been reported in cases of endogenous bacterial endophthalmitis. In patients with relatively clear media with less severe vitreous involvement, other findings like subretinal, preretinal, flame-shaped hemorrhage, Roth spots, cotton wool spots, retinal and choroidal detachment can also be documented. MRSA has been associated with a high rate of retinal detachment when the presentation is delayed more than two weeks after the onset of symptoms.[33]
EE can be broadly classified into three categories based on ocular signs and symptoms, as detailed below.[29] Petit et al. earlier has proposed a classification for fungal EE.[34]
Table
S. No Involvement
Evaluation
Apart from clinical diagnostic, EE is confirmed by imaging and lab investigations. Every case warrants a detailed slit lamp and dilated fundus evaluation. Sometimes the diagnosis is delayed since the clinical picture can have a lot of differentials, and multiple assessments are required before reaching a conclusive diagnosis. The prime importance should be given to systemic evaluation and management.
Imaging Modalities
B Scan Ultrasound
B scan ultrasound is an essential diagnostic modality to pick up vitritis, membranes, exudates in the vitreous cavity, retinal detachment, choroidal detachment, and choroidal abscess. The exudates in the vitreous cavity are present as echoes on the B scan. EE is also known to cause a choroidal abscess, which appears as dome-shaped elevation on B scan similar to choroidal detachment.[35]
Optical Coherence Tomography
Optical coherence tomography (OCT) is an important imaging modality to delineate the retinal and subretinal space. OCT can help localize the exudates within subretinal layers, lesions of the retinal pigment elevation, retinal edema, vitreous cells and exudates, and choroidal involvement.[36]
Anterior Chamber and Vitreous Tap
An anterior chamber aqueous tap of 0.1-0.2 ml can be performed with a 23 G needle. Vitreous tap of 1-2 ml is performed with the help of a 25 G needle. The sample can also be obtained while performing vitrectomy and subjected to culture, sensitivity, and histopathological examination. Lingappan et al. proved that samples obtained with vitrectomy showed a better culture positivity (92%) than vitreous tap (44%). Similarly, Zhang et al. showed that the sample obtained needle biopsy had a lower diagnostic yield than vitrectomy as the vitrectomy samples were closer to the retinal surface.[37]
Polymerase Chain Reaction
The samples obtained through aqueous and vitreous tap can be subjected to a real-time polymerase chain reaction (RT-PCR), with very high sensitivity and specificity. RTPCR has proven high sensitivity and specificity for fungi as per the analysis by Sugita et al. In culture-negative cases of EE; it has proven to be a better diagnostic modality for detecting microorganisms. PCR results are obtained within 90 minutes, with better diagnostic yield than culture results with no risk of contamination. The major limitation of PCR is that an antibiotic susceptibility pattern cannot be obtained through this.[38]
Blood Culture
Blood culture is an important diagnostic modality for the diagnosis of systemic infection. Three consecutive samples can be obtained on three days under aseptic precautions for a better diagnostic yield. Besides blood culture, it is vital to obtain urine culture to identify occult microorganisms. According to a few previous studies, blood culture has shown a higher culture positivity than the vitreous sample, probably due to the sample volume obtained. Culture at extraocular sites has yielded a 21-100% positivity rate as per previous reports.[17]
Upcoming Modalities
2-Deoxy-2-[18F] Fluoro-Dglucose Positron Emission Tomography/Computed Tomography Scan
Mehta et al. recently documented using 2-Deoxy-2-[18F] fluoro-D-glucose positron emission tomography/computed tomography scan for systemic assessment of patients with presumed fungal EE in patients with COVID-19 infection.[39]
Treatment / Management
Endogenous endophthalmitis is an emergency, and prompt diagnosis and meticulous management are required to safeguard vision in these cases. The treatment is governed by the stage of the disease, offending microorganism, mode and route of infection, and systemic status of the patient. A flowchart of how to manage endogenous endophthalmitis is depicted below.
Management Protocol for Bacterial Endogenous Endophthalmitis
Systemic Antibacterials
Bacterial EE warrants treatment of systemic infection with antibiotics. Once the blood culture results are available, systemic antibiotics can be initiated. Moreover, severe ocular infection warrants the addition of intravitreal injections. Non-resolving cases with rapidly progressive bacteremia will require pars plana vitrectomy.
Local Therapy
Once infection EE is suspected, a sample for culture and sensitivity should be obtained, and intravitreal antibiotics should be initiated. Early intravitreal antibiotics should be initiated within 24 hours of presentation as they have been reported to have a better and favorable outcome. Before the etiology is established, broad-spectrum intravitreal antibiotics should be administered. Vancomycin 1mg/0.1 ml and cefazolin 2.25 mg/0.1 ml covers gram-positive bacteria, and 2.25 mg/0.1 ml ceftazidime or 0.4 mg/0.1 ml amikacin are the drug of choice for gram-negative bacteria. Vancomycin is the drug of choice for MRSA. However, there have been recent reports of vancomycin resistance staphylococcus aureus (VRSA) also. The drugs commonly used for VRSA are daptomycin 200 ug/0.1 ml and quinupristin/dalfopristin 0.4 mg/0.1 ml. Fluoroquinolones are a broad spectrum with gram-positive and negative coverage.
Once the culture results are available, targeted therapy should be started for rapid recovery. Prompt diagnosis and targeted treatment are essential for a final better outcome. Antibiotics should be used cautiously in pregnant and lactating mothers. Penicillin group of drugs, cephalosporins, and erythromycin can be used safely in these patients. Cartilage abnormalities have been reported secondary to fluoroquinolones; hence they should not be used as the first line in pregnant women.[40][41][11]
Management Protocol for Endogenous Fungal Endophthalmitis
Candida Endogenous Endophthalmitis
The drug of choice for candida EE is intravitreal amphotericin or voriconazole. Amphotericin B (AMB) deoxycholate is given in the dose of 5 to 10 ug/0.1 ml dextrose or sterile saline. The patient should be reassessed after 48 hours, and if needed, the dose can be repeated. AMB has been reported to cause nephrotoxicity, hypotension, and arrhythmia. Hence the patient should be closely monitored for these side effects. Intravitreal voriconazole is administered in a dose of 100-200 ug/0.1 ml sterile saline.[42][43]
The other alternative recommended by the infectious disease society of America (IDSA) is fluconazole. The recommended dose is 400-800 mg per day. Hamada et al. recommended fluconazole as the first-line therapy because of its better side effect profile than AMB. In severe cases with non-resolving vitritis, pars plana vitrectomy is the treatment of choice. The treatment duration depends on the clinical picture, but a minimum of 6 weeks is recommended.[44][45]
Endogenous Endophthalmitis Secondary to Aspergillus, Paecilomyces, and Fusarium
The drugs implicated are voriconazole, AMB, and fluconazole. Voriconazole has proven high efficacy of approximately 100% in these cases. Pars plana vitrectomy with lensectomy or intraocular lens removal should be performed in non-resolving cases. In bedridden patients, those who are severely immunocompromised or unable to tolerate surgery, systemic antifungal therapy should be initiated.[46][47][48]
Pars Plana Vitrectomy
Pars plana vitrectomy (PPV) is the treatment of choice for non-resolving vision-threatening bacterial and fungal EE cases. PPV serves both diagnostic and therapeutic purposes. Pre-surgery or intraoperatively intravitreal drugs can be administered to reduce the microorganism load. Vitrectomy helps to reduce the microorganism load in the posterior segment and assists in rapid recovery. Vitrectomy is based on the vitreoretinal surgeon's discretion.
Early vitrectomy has been reported to cause a reduced incidence of retinal detachment and has a better globe salvage rate. The incidence of enucleation and evisceration is also reduced. Sato et al. suggested early vitrectomy in Candida EE cases. Zhang et al. found better visual outcomes in patients who were subjected to early vitrectomy. Yoon et al. suggested early PPV in Klebsiella endophthalmitis, which may result in better visual effects.[49]
Corticosteroids
The role of intravitreal and systemic steroid use in EE is controversial, and there are no set guidelines for the same. Bacterial endotoxins and profound inflammation is known to cause retinal pigment epithelial damage and necrosis. Steroids combat inflammation, alleviate leucocyte migration, reduce cytokine storm and stabilize the blood-retinal barrier. Jackson et al. showed that patients with EE treated with steroids resulted in a better visual outcome. In contrast, Shuwan et al. showed no significant benefit with the use of steroids. Lindstedt et al., in their multicentric randomized trial, showed that intravitreal dexamethasone had no added benefit. Hence, in a nutshell, steroids should be used with caution in EE patients.[50][51]
Differential Diagnosis
Infectious
- Cytomegalovirus
- Herpes simplex virus
- Varicella-zoster virus
- Epstein bar virus
- Disseminated toxoplasmosis
- Disseminated viral retinitis
- Tuberculosis
- Lyme's
Non-infectious
- Vogt-Koyanagi-Harada syndrome
- Sarcoidosis
- Behcet syndrome[55]
- Idiopathic
- Toxic anterior segment syndrome
- Sympathetic ophthalmia[56]
- Juvenile idiopathic arthritis
- Posterior scleritis
Postoperative endophthalmitis (after any surface or intraocular surgery)
Masquerade
- Leukemia
- Lymphoma
- Retinoblastoma
- Choroidal melanoma
- Tumour necrosis with inflammation
Intraocular foreign body
White dot syndromes
- Punctate inner choroidopathy
- Serpiginous choroidopathy
- Multifocal choroiditis and panuveitis
- Subretinal fibrosis and uveitis
- Multiple evanescent white dot syndrome
- Acute posterior multifocal placoid pigment epitheliopathy
- Birdshot chorioretinopathy
Asteroid Hyalosis
Disseminated Intravascular Coagulation
Thrombotic Thrombocytopenic Purpura
Prognosis
The prognosis of endogenous endophthalmitis depends on the presentation time, clinical manifestation, the extent of vitreous involvement, and virulence of the offending microorganism. It's a complex entity to diagnose, and due to various comorbidities, it is often picked up late. However, studies have proven that yeasts have the best prognosis, followed by bacteria followed by molds which have the worst prognosis.
Patients who present late and in whom diagnosis and treatment are delayed often land up with complete vision loss, and the prognosis is poor in these cases. The patients requiring vitreous tap are at risk for retinal detachment, and the prognosis is guarded in these cases. Zenith et al., in their analysis, showed that bacterial EE had a poor outcome and prognosis compared to fungal EE and these cases usually require enucleation and evisceration.[17] Yonekawa et al., in their study, proven that prompt diagnosis and treatment was associated with 64% of patients having visual outcomes better than counting finger (CF) in bacterial EE.[41]
Similarly, Itoh et al. showed that early and aggressive vitrectomy within two weeks of diagnosis results in good final outcomes, and the prognosis is good in these cases.[57] Among the offending microorganisms, it has been reported that MRSA EE patients have a poor prognosis and high mortality. However, few studies have proven variable association between the two. Connell et al., in their analysis, showed that the majority of patients with Klebsiella EE required vitrectomy.[58] Ang et al. found that hypopyon in Klebsiella EE can be a prognostic predictor.[40]
Complications
Postoperative and Rehabilitation Care
All patients with EE should be admitted to the hospital, and close monitoring of systemic and ocular parameters is mandated in these cases. Patients requiring intravitreal injections should be evaluated daily for improvement in clinical picture and need for further injections. In case of worsening EE, the surgeon's discretion makes a decision for pars plana vitrectomy. Post vitrectomy, the patient should be started on topical steroids prednisolone 1% or dexamethasone 0.1% in tapering doses 8/7/6/5/4/3/2/1 every week, 5% homatropine two times per day, topical 0.5% timolol two times per day, and oral antibiotics and anti-inflammatory. The patient should be followed up closely to assess the clinical picture and rule out any complications.[63]
Consultations
Endogenous endophthalmitis is a severe sight-threatening emergency, and all patients with suspected EE must be referred and evaluated by a vitreoretinal surgeon. The vitreoretinal surgeon has a vital role to play in the diagnosis and management of these cases.
Deterrence and Patient Education
The patients must be explained the serious nature of the pathology and warning signs and symptoms like pain, redness, swelling, sudden reduction in visual acuity, and photophobia. The patients must be explained that the condition will require hospital admission and intensive treatment in topical and oral drugs, intravitreal injection, and the need for surgery. The patient should be explained regarding visual prognosis and to have realistic expectations.[17]
Enhancing Healthcare Team Outcomes
Endogenous endophthalmitis is usually seen in patients with diabetes mellitus, immunosuppression, and other systemic pathologies. Nursing staff must keep a check on blood sugar, temperature, and other vital parameters. The treating physician must be highly suspicious of EE if the patient reports pain, redness, and sudden vision loss. Patients will all these symptoms must be referred to a vitreoretinal (VR) surgeon for meticulous management. The VR surgeon also plays a vital role in counseling and explaining the prognosis of the pathology to the patient. Nursing staff also plays a crucial role in counseling and regularly administering drugs to the patients in collaboration with the pharmacist. The patient's final visual and systemic outcome depends on the multidisciplinary approach by the physician, pharmacist, nursing staff, and the vitreoretinal surgeons.
Review Questions
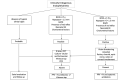
Figure
Flowchart depicting management of endogenous endophthalmitis Contributed by Dr. Bharat Gurnani, MBBS, DNB, FCRS, FICO, MRCS Ed, MNAMS
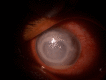
Figure
Slit lamp image of the patient depicting Klebsiella endogenous endophthalmitis in a patient with chronic urinary tract infection Contributed by Dr. Bharat Gurnani, MBBS, DNB, FCRS, FICO, MRCS Ed, MNAMS

Figure
Slit lamp image of the patient depicting corneal melt, full thickness corneal infiltrate in a patient with pseudomonas endogenous endophthalmitis Contributed by Dr. Bharat Gurnani, MBBS, DNB, FCRS, FICO, MRCS Ed, MNAMS

Figure
Slit lamp image of the patient depicting 8x8 mm full thickness corneal infiltrate with corneal melt, corneal abscess in a patient with staphylococcal endogenous endophthalmitis Contributed by Dr. Bharat Gurnani, MBBS, DNB, FCRS, FICO, MRCS Ed, MNAMS
References
- 1.
- Novosad BD, Callegan MC. Severe bacterial endophthalmitis: towards improving clinical outcomes. Expert Rev Ophthalmol. 2010 Oct;5(5):689-698. [PMC free article: PMC3092298] [PubMed: 21572565]
- 2.
- Lynn WA, Lightman S. The eye in systemic infection. Lancet. 2004 Oct 16-22;364(9443):1439-50. [PubMed: 15488221]
- 3.
- Kernt M, Kampik A. Endophthalmitis: Pathogenesis, clinical presentation, management, and perspectives. Clin Ophthalmol. 2010 Mar 24;4:121-35. [PMC free article: PMC2850824] [PubMed: 20390032]
- 4.
- Schiedler V, Scott IU, Flynn HW, Davis JL, Benz MS, Miller D. Culture-proven endogenous endophthalmitis: clinical features and visual acuity outcomes. Am J Ophthalmol. 2004 Apr;137(4):725-31. [PubMed: 15059712]
- 5.
- Sharma S, Padhi TR, Basu S, Kar S, Roy A, Das T. Endophthalmitis patients seen in a tertiary eye care centre in Odisha: a clinico-microbiological analysis. Indian J Med Res. 2014 Jan;139(1):91-8. [PMC free article: PMC3994746] [PubMed: 24604043]
- 6.
- Jackson TL, Paraskevopoulos T, Georgalas I. Systematic review of 342 cases of endogenous bacterial endophthalmitis. Surv Ophthalmol. 2014 Nov-Dec;59(6):627-35. [PubMed: 25113611]
- 7.
- Sridhar J, Flynn HW, Kuriyan AE, Miller D, Albini T. Endogenous fungal endophthalmitis: risk factors, clinical features, and treatment outcomes in mold and yeast infections. J Ophthalmic Inflamm Infect. 2013 Sep 20;3(1):60. [PMC free article: PMC3848556] [PubMed: 24053550]
- 8.
- Hu CC, Ho JD, Lou HY, Keller JJ, Lin HC. A one-year follow-up study on the incidence and risk of endophthalmitis after pyogenic liver abscess. Ophthalmology. 2012 Nov;119(11):2358-63. [PubMed: 22817832]
- 9.
- Rudež LK, Petrović I, Bulić K, Mandić JJ. A rare case of devastating Clostridium septicum endogenous endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2022 Jun;260(6):2061-2064. [PubMed: 34724111]
- 10.
- Yue Q, Zhang Q, Wang Y, He X, Ding K, Wang X, Xi H, Wang L, Zhang Y, Wu C. Complete Genome Sequencing and Comparative Analysis of Citrobacter koseri CKNJ, a Strain Isolated from a Patient with Endogenous Endophthalmitis. Jpn J Infect Dis. 2022 Mar 24;75(2):148-155. [PubMed: 34470967]
- 11.
- Sahu C, Kumar K, Sinha MK, Venkata A, Majji AB, Jalali S. Review of endogenous endophthalmitis during pregnancy including case series. Int Ophthalmol. 2013 Oct;33(5):611-8. [PubMed: 23263732]
- 12.
- Moshfeghi AA, Charalel RA, Hernandez-Boussard T, Morton JM, Moshfeghi DM. Declining incidence of neonatal endophthalmitis in the United States. Am J Ophthalmol. 2011 Jan;151(1):59-65.e1. [PubMed: 20970776]
- 13.
- Durand ML. Endophthalmitis. Clin Microbiol Infect. 2013 Mar;19(3):227-34. [PMC free article: PMC3638360] [PubMed: 23438028]
- 14.
- Lamaris GA, Esmaeli B, Chamilos G, Desai A, Chemaly RF, Raad II, Safdar A, Lewis RE, Kontoyiannis DP. Fungal endophthalmitis in a tertiary care cancer center: a review of 23 cases. Eur J Clin Microbiol Infect Dis. 2008 May;27(5):343-7. [PubMed: 18183439]
- 15.
- Keyashian K, Malani PN. Endophthalmitis associated with intravenous drug use. South Med J. 2007 Dec;100(12):1219-20. [PubMed: 18090965]
- 16.
- Cheng HH, Ding Y, Wu M, Tang CC, Zhang RJ, Lin XF, Xu JT. Endogenous aspergillus endophthalmitis after kidney transplantation. Int J Ophthalmol. 2011;4(5):567-71. [PMC free article: PMC3340725] [PubMed: 22553722]
- 17.
- Wu ZH, Chan RP, Luk FO, Liu DT, Chan CK, Lam DS, Lai TY. Review of Clinical Features, Microbiological Spectrum, and Treatment Outcomes of Endogenous Endophthalmitis over an 8-Year Period. J Ophthalmol. 2012;2012:265078. [PMC free article: PMC3594912] [PubMed: 23533699]
- 18.
- de Lima LM, Cecchetti SA, Cecchetti DF, Arroyo D, Romão EA, Dantas M, Neto MM. Endophthalmitis: a rare but devastating metastatic bacterial complication of hemodialysis catheter-related sepsis. Ren Fail. 2012;34(1):119-22. [PubMed: 22017567]
- 19.
- Noyola DE, Bohra L, Paysse EA, Fernandez M, Coats DK. Association of candidemia and retinopathy of prematurity in very low birthweight infants. Ophthalmology. 2002 Jan;109(1):80-4. [PubMed: 11772584]
- 20.
- Basu S, Kumar A, Kapoor K, Bagri NK, Chandra A. Neonatal endogenous endophthalmitis: a report of six cases. Pediatrics. 2013 Apr;131(4):e1292-7. [PubMed: 23478867]
- 21.
- Bilgic A, Sudhalkar A, Gonzalez-Cortes JH, March de Ribot F, Yogi R, Kodjikian L, Mathis T. ENDOGENOUS ENDOPHTHALMITIS IN THE SETTING OF COVID-19 INFECTION: A Case Series. Retina. 2021 Aug 01;41(8):1709-1714. [PubMed: 33734193]
- 22.
- Agarwal M, Sachdeva M, Pal S, Shah H, Kumar R M, Banker A. Endogenous Endophthalmitis A Complication of COVID-19 Pandemic: A Case Series. Ocul Immunol Inflamm. 2021 May 19;29(4):726-729. [PubMed: 34255587]
- 23.
- Gurnani B, Kaur K. Impact of the COVID-19 pandemic on clinical ophthalmology. 2021 Jan & FebIndian J Med Res. 153(1 & 2):199-200. [PMC free article: PMC8184090] [PubMed: 33473015]
- 24.
- Tsai AS, Lee SY, Jap AH. An unusual case of recurrent endogenous Klebsiella endophthalmitis. Eye (Lond). 2010 Oct;24(10):1630-1. [PubMed: 20559330]
- 25.
- Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003 Jul-Aug;48(4):403-23. [PubMed: 12850229]
- 26.
- Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM. Endogenous endophthalmitis: an 18-year review of culture-positive cases at a tertiary care center. Medicine (Baltimore). 2003 Mar;82(2):97-105. [PubMed: 12640186]
- 27.
- Greenwald MJ, Wohl LG, Sell CH. Metastatic bacterial endophthalmitis: a contemporary reappraisal. Surv Ophthalmol. 1986 Sep-Oct;31(2):81-101. [PubMed: 3541265]
- 28.
- Samiy N, D'Amico DJ. Endogenous fungal endophthalmitis. Int Ophthalmol Clin. 1996 Summer;36(3):147-62. [PubMed: 8989607]
- 29.
- Chee SP, Jap A. Endogenous endophthalmitis. Curr Opin Ophthalmol. 2001 Dec;12(6):464-70. [PubMed: 11734687]
- 30.
- Lingappan A, Wykoff CC, Albini TA, Miller D, Pathengay A, Davis JL, Flynn HW. Endogenous fungal endophthalmitis: causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol. 2012 Jan;153(1):162-6.e1. [PubMed: 21917234]
- 31.
- Agarwal M, Biswas J, Mathur U, Sijwali MS, Singh AK. Aspergillus iris granuloma in a young male: a case report with review of literature. Indian J Ophthalmol. 2007 Jan-Feb;55(1):73-4. [PubMed: 17189896]
- 32.
- Oude Lashof AM, Rothova A, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Schlamm HT, Oborska IT, Rex JH, Kullberg BJ. Ocular manifestations of candidemia. Clin Infect Dis. 2011 Aug 01;53(3):262-8. [PubMed: 21765074]
- 33.
- Khan A, Okhravi N, Lightman S. The eye in systemic sepsis. Clin Med (Lond). 2002 Sep-Oct;2(5):444-8. [PMC free article: PMC4953086] [PubMed: 12448593]
- 34.
- Tanaka M, Kobayashi Y, Takebayashi H, Kiyokawa M, Qiu H. Analysis of predisposing clinical and laboratory findings for the development of endogenous fungal endophthalmitis. A retrospective 12-year study of 79 eyes of 46 patients. Retina. 2001;21(3):203-9. [PubMed: 11421007]
- 35.
- Rodrigues IA, Jackson TL. A high-definition view of endogenous fungal endophthalmitis. Lancet Infect Dis. 2014 Apr;14(4):358. [PubMed: 24670628]
- 36.
- Cho M, Khanifar AA, Chan RV. Spectral-domain optical coherence tomography of endogenous fungal endophthalmitis. Retin Cases Brief Rep. 2011 Spring;5(2):136-40. [PubMed: 25389883]
- 37.
- Palexas GN, Green WR, Goldberg MF, Ding Y. Diagnostic pars plana vitrectomy report of a 21-year retrospective study. Trans Am Ophthalmol Soc. 1995;93:281-308; discussion 308-14. [PMC free article: PMC1312062] [PubMed: 8719683]
- 38.
- Sowmya P, Madhavan HN. Diagnostic utility of polymerase chain reaction on intraocular specimens to establish the etiology of infectious endophthalmitis. Eur J Ophthalmol. 2009 Sep-Oct;19(5):812-7. [PubMed: 19787602]
- 39.
- Mehta S, Nagvekar V, Gupta G. Utility of 2-Deoxy-2-[18F]fluoro-Dglucose positron emission tomography/computed tomography scan in the systemic evaluation of patients with post-COVID-19 endogenous presumed fungal endophthalmitis. Indian J Ophthalmol. 2021 Oct;69(10):2873-2874. [PMC free article: PMC8597434] [PubMed: 34571657]
- 40.
- Ang M, Jap A, Chee SP. Prognostic factors and outcomes in endogenous Klebsiella pneumoniae endophthalmitis. Am J Ophthalmol. 2011 Feb;151(2):338-44.e2. [PubMed: 21168820]
- 41.
- Yonekawa Y, Chan RV, Reddy AK, Pieroni CG, Lee TC, Lee S. Early intravitreal treatment of endogenous bacterial endophthalmitis. Clin Exp Ophthalmol. 2011 Nov;39(8):771-8. [PubMed: 22050564]
- 42.
- Barza M. Treatment options for candidal endophthalmitis [editoria; comment]. Clin Infect Dis. 1998 Nov;27(5):1134-6. [PubMed: 9827258]
- 43.
- Martínez-Vázquez C, Fernández-Ulloa J, Bordón J, Sopeña B, de la Fuente J, Ocampo A, Rubianes M. Candida albicans endophthalmitis in brown heroin addicts: response to early vitrectomy preceded and followed by antifungal therapy. Clin Infect Dis. 1998 Nov;27(5):1130-3. [PubMed: 9827257]
- 44.
- Breit SM, Hariprasad SM, Mieler WF, Shah GK, Mills MD, Grand MG. Management of endogenous fungal endophthalmitis with voriconazole and caspofungin. Am J Ophthalmol. 2005 Jan;139(1):135-40. [PubMed: 15652837]
- 45.
- Pappas PG, Kauffman CA, Andes D, Benjamin DK, Calandra TF, Edwards JE, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD., Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009 Mar 01;48(5):503-35. [PMC free article: PMC7294538] [PubMed: 19191635]
- 46.
- Sen P, Gopal L, Sen PR. Intravitreal voriconazole for drug-resistant fungal endophthalmitis: case series. Retina. 2006 Oct;26(8):935-9. [PubMed: 17031296]
- 47.
- Marangon FB, Miller D, Giaconi JA, Alfonso EC. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am J Ophthalmol. 2004 May;137(5):820-5. [PubMed: 15126145]
- 48.
- Jørgensen JS, Prause JU, Kiilgaard JF. Bilateral endogenous Fusarium solani endophthalmitis in a liver-transplanted patient: a case report. J Med Case Rep. 2014 Mar 24;8:101. [PMC free article: PMC3978080] [PubMed: 24661421]
- 49.
- Smith SR, Kroll AJ, Lou PL, Ryan EA. Endogenous bacterial and fungal endophthalmitis. Int Ophthalmol Clin. 2007 Spring;47(2):173-83. [PubMed: 17450017]
- 50.
- Lindstedt EW, Bennebroek CA, van der Werf DJ, Veckeneer M, Norel AO, Nielsen CC, Wubbels RJ, van Dissel JT, van Meurs JC. A prospective multicenter randomized placebo-controlled trial of dexamethasone as an adjuvant in the treatment of postoperative bacterial endophthalmitis: interim safety analysis of the study drug and analysis of overall treatment results. Graefes Arch Clin Exp Ophthalmol. 2014 Oct;252(10):1631-7. [PubMed: 25107542]
- 51.
- Lee S, Um T, Joe SG, Hwang JU, Kim JG, Yoon YH, Lee JY. Changes in the clinical features and prognostic factors of endogenous endophthalmitis: fifteen years of clinical experience in Korea. Retina. 2012 May;32(5):977-84. [PubMed: 22105504]
- 52.
- Gurnani B, Kaur K. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jul 17, 2023. Pythium Keratitis. [PubMed: 34424645]
- 53.
- Gurnani B, Christy J, Narayana S, Rajkumar P, Kaur K, Gubert J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian J Ophthalmol. 2021 May;69(5):1095-1101. [PMC free article: PMC8186601] [PubMed: 33913840]
- 54.
- Gurnani B, Narayana S, Christy J, Rajkumar P, Kaur K, Gubert J. Successful management of pediatric pythium insidiosum keratitis with cyanoacrylate glue, linezolid, and azithromycin: Rare case report. Eur J Ophthalmol. 2022 Sep;32(5):NP87-NP91. [PubMed: 33779337]
- 55.
- Balamurugan S, Das D, Hasanreisoglu M, Toy BC, Akhter M, Anuradha VK, Anthony E, Gurnani B, Kaur K. Interleukins and cytokine biomarkers in uveitis. Indian J Ophthalmol. 2020 Sep;68(9):1750-1763. [PMC free article: PMC7690463] [PubMed: 32823391]
- 56.
- Paulbuddhe V, Addya S, Gurnani B, Singh D, Tripathy K, Chawla R. Sympathetic Ophthalmia: Where Do We Currently Stand on Treatment Strategies? Clin Ophthalmol. 2021;15:4201-4218. [PMC free article: PMC8542579] [PubMed: 34707340]
- 57.
- Itoh M, Ikewaki J, Kimoto K, Itoh Y, Shinoda K, Nakatsuka K. Two Cases of Endogenous Endophthalmitis Caused by Gram-Positive Bacteria with Good Visual Outcome. Case Rep Ophthalmol. 2010 Sep 21;1(2):56-62. [PMC free article: PMC2988850] [PubMed: 21103197]
- 58.
- Connell PP, O'Neill EC, Fabinyi D, Islam FM, Buttery R, McCombe M, Essex RW, Roufail E, Clark B, Chiu D, Campbell W, Allen P. Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye (Lond). 2011 Jan;25(1):66-72. [PMC free article: PMC3144637] [PubMed: 20966972]
- 59.
- Christy J, Jain N, Gurnani B, Kaur K. Twinkling Eye -A Rare Presentation in Neovascular Glaucoma. J Glaucoma. 2019 May 23; [PubMed: 31135586]
- 60.
- Gurnani B, Kaur K, Gireesh P. Rare Coexistence of Bilateral Congenital Sutural and Cortical Blue Dot Cataracts. J Pediatr Ophthalmol Strabismus. 2020 Jan 01;57(1):68. [PubMed: 31972045]
- 61.
- Balamurugan S, Gurnani B, Kaur K, Gireesh P, Narayana S. Traumatic intralenticular abscess-What is so different? Indian J Radiol Imaging. 2020 Jan-Mar;30(1):92-94. [PMC free article: PMC7240887] [PubMed: 32476758]
- 62.
- Gurnani B, Kaur K, Sekaran S. First case of coloboma, lens neovascularization, traumatic cataract, and retinal detachment in a young Asian female. Clin Case Rep. 2021 Sep;9(9):e04743. [PMC free article: PMC8405537] [PubMed: 34484773]
- 63.
- Lim HW, Shin JW, Cho HY, Kim HK, Kang SW, Song SJ, Yu HG, Oh JR, Kim JS, Moon SW, Chae JB, Park TK, Song Y. Endogenous endophthalmitis in the Korean population: a six-year retrospective study. Retina. 2014 Mar;34(3):592-602. [PubMed: 24056527]
Disclosure: Bharat Gurnani declares no relevant financial relationships with ineligible companies.
Disclosure: Kirandeep Kaur declares no relevant financial relationships with ineligible companies.
- Continuing Education Activity
- Introduction
- Etiology
- Epidemiology
- Pathophysiology
- History and Physical
- Evaluation
- Treatment / Management
- Differential Diagnosis
- Prognosis
- Complications
- Postoperative and Rehabilitation Care
- Consultations
- Deterrence and Patient Education
- Enhancing Healthcare Team Outcomes
- Review Questions
- References
- [Chinese expert consensus on the diagnosis and treatment of pneumonia in the elderly (2024 Edition)].[Zhonghua Jie He He Hu Xi Za Zh...][Chinese expert consensus on the diagnosis and treatment of pneumonia in the elderly (2024 Edition)].Respiratory Branch of Chinese Geriatrics Society. Zhonghua Jie He He Hu Xi Za Zhi. 2025 Jan 12; 48(1):18-34.
- Lens Abscess.[StatPearls. 2025]Lens Abscess.Kaur K, Gurnani B. StatPearls. 2025 Jan
- EMS Management of Eye Injuries.[StatPearls. 2025]EMS Management of Eye Injuries.Walsh A, Lewis K. StatPearls. 2025 Jan
- Review FBN1-Related Marfan Syndrome.[GeneReviews(®). 1993]Review FBN1-Related Marfan Syndrome.Dietz H. GeneReviews(®). 1993
- Review Retinoblastoma.[GeneReviews(®). 1993]Review Retinoblastoma.Lohmann DR, Gallie BL. GeneReviews(®). 1993
- Endogenous Endophthalmitis - StatPearlsEndogenous Endophthalmitis - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
See more...
