This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
The colorectal cancer (CRC) screening rate among Hispanics in the United States is low. There is a need for research to develop effective, evidence-based intervention methods that can increase CRC screening among eligible individuals in this population.
Objectives:
We designed the current study to test the impact of a mailed standard intervention (SI; an educational brochure, an immunochemical stool blood test [SBT], instructions for scheduling a screening colonoscopy, and a reminder) vs the combination of a mailed standard intervention plus telephone decision support and navigation intervention (DSNI) on CRC screening adherence among Hispanic primary care patients. Additionally, the study aimed to assess DSNI vs SI effects on participant screening decision stage and on knowledge and perceptions related to screening.
Methods:
Between October 2014 and March 2017 our research team identified Hispanic patients, aged 50 to 75 years who were not up to date with CRC screening, in 5 primary care practices that were part of the Lehigh Valley Health Network in southeastern Pennsylvania. The research team randomized, consented, and surveyed patients either to an SI group (n = 203) or a DSNI group (n = 197). We provided SI group materials to both study groups in English and Spanish. DSNI group participants also received a telephone call from a bilingual patient navigator who reviewed the screening materials, verified the participant's preferred CRC screening test, and assessed the individual's likelihood of completing his or her preferred test. Using this information, the patient navigator helped DSNI group participants develop and implement a test-specific screening plan. We administered a 6-month telephone survey and conducted a 12-month medical records review for all participants. We assessed the primary outcome—screening adherence at 12 months postrandomization—using multivariable logistic regression. We also performed multivariable analyses to assess intervention impact on readiness to screen, as measured by change in decision stage, and on knowledge and perceptions about screening at 6 months postrandomization.
Results:
Overall CRC screening adherence was significantly and substantially higher in the DSNI group than in the SI group (78% vs 43%, respectively; adjusted odds ratio [OR], 4.83; 95% CI, 3.08-7.58; P < .001). Compared with the SI group, the DSNI group also displayed a higher SBT screening rate (57% vs 43%, respectively; OR, 4.20; 95% CI, 2.63-6.70; P < .001), a higher colonoscopy screening rate (20% vs 6%, respectively; OR, 8.79; 95% CI, 4.13-8.74; P < .001), and greater “forward” change in screening decision stage (79% vs 50%, respectively; OR, 4.91; CI, 2.55-9.47; P < .001). Participant knowledge and perceptions related to CRC screening did not differ significantly by study group (P = .862 and .880, respectively).
Conclusions:
Among Hispanic primary care patients, the DSNI strategy achieved significantly greater positive effects on CRC screening adherence, decision stage, and test-specific screening adherence.
Limitations and subpopulation considerations:
The generalizability of study findings may be limited, as we conducted the study with patients from 5 primary care practices in a single health system; participants in the study may differ from Hispanic primary care patients in other geographic regions and primary care patient populations. Moreover, the effectiveness of DSNI delivery might have been influenced by unique characteristics of the patient navigator.
Background
It is well-known that colorectal cancer (CRC) screening can find colorectal adenomas, which can be removed before they progress to CRC, and can detect early-stage CRC, when the disease can be cured. Guidelines of the US Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) recommend CRC screening for people aged 50 to 75 years and at average risk for CRC.1,2 Having a colonoscopy every 10 years or annual stool blood testing (SBT) are the CRC screening tests most frequently referred by primary care physicians.3 Overall, the CRC screening rate in the United States has been reported to be 63%, but the average screening rate among Hispanics is substantially lower.4
CRC is the second-leading cause of cancer deaths among Hispanics in the United States.4 Recent reports show that Hispanics in some sections of the country are more likely than non-Hispanic Whites to be diagnosed with late-stage CRC.5 Unfortunately, lower CRC screening rates translate into higher stage-specific mortality among Hispanics compared with non-Hispanic Whites, and the CRC mortality rate among Hispanics will soon be greater than that of non-Hispanic Whites.6 The disparities in CRC screening and mortality between Hispanics and non-Hispanic Whites persist, even when adjusting for education, income, and insurance status.7,8
The National Academy of Medicine (NAM), formerly the Institute of Medicine, has encouraged research that addresses racial and ethnic disparities in health care.9 The NAM also has highlighted the need to compare the effectiveness of patient decision support tools, to compare the effectiveness of different strategies to engage and retain patients in care, and to delineate barriers to care, especially for members of populations that experience health disparities. In 2011, the Agency for Health Research and Quality (AHRQ) also called for research on the implementation of interventions that aim to reduce health disparities in priority populations, including racial and ethnic groups.10 Our study was responsive to both NAM and AHRQ priorities.
Achieving high CRC screening rates in primary care can have a substantial, positive impact on CRC-related mortality and survival, along with related disparities, and can reduce economic costs associated with the disease.11 In an analysis, Meester et al12 estimated that by increasing the CRC screening rate from 60% to 100%, a 63% reduction in CRC mortality could be achieved. In economic terms, it has been projected that increased CRC screening could result in almost $15 billion in savings by 2020.13
Literature Review
Effective, patient-centered interventions are needed, however, to increase CRC screening14 and reduce the burden of this disease among Hispanics.4,7-15 Systematic reviews by Robie et al16 and Gonzalez et al 17 show that few randomized controlled trials of different interventions intended to increase CRC screening among Hispanics have been reported in the literature. Another recent systematic review18 reported that tailored patient education combined with patient navigation services had a modest, positive effect on adherence to CRC screening. However, only 8 studies in the review included a substantial number of Hispanics, and findings related to Hispanic patients were not presented separately. Furthermore, few studies have intervened with Hispanic patients outside the context of an office visit, and none have assessed the impact of decision support and navigation on CRC screening rates in this population.
Mailed Contacts, Message Tailoring, and Preference
Recent publications including a meta-analysis, a systematic review, and an integrative review, have found that generic patient contacts (eg, print materials, mailed SBTs, reminder mailings) have produced a modest, positive impact on CRC screening, especially SBT screening.19-21 Rawl et al21 also reported that mailed patient-oriented, personalized print education materials may have a positive impact on CRC screening, especially in minority populations. Gonzalez et al17 found mailed patient materials had a modest positive impact on SBT screening among Hispanic primary care patients. Research on message tailoring in CRC screening in the general population is limited and almost nonexistent in Hispanic populations.
Message tailoring refers to the delivery of educational messages that address personal barriers (ie, cognitive needs and psychosocial concerns related to specific behaviors) and serves to minimize or remove psychosocial obstacles to action. Educational message tailoring has been used successfully to promote smoking cessation; reduce dietary fat intake; and increase cholesterol testing, physical activity, and mammography use.22-30
To reduce disparities, Kreuter et al31,32 and Powe33-35 have encouraged the delivery of tailored messages that address barriers relevant to population subgroups. Only a few research studies have reported on the use of message tailoring to address psychosocial barriers to CRC screening.36-38 This type of message tailoring intervention has generated only modest effects at best. The use of message tailoring based on personal preference for different CRC screening tests may be more successful. However, no studies have been reported on the use of such methods among Hispanics.
Wolf et al39 elicited CRC screening test preferences among minority patients attending an urban health care clinic and found survey respondents commonly reported a preference for SBT screening. Hawley et al40 reported that race was associated with differences in screening test preference among patients who visited a primary care practice. The literature also suggests that personal preference not only influences health behavior but may also be related to observed disparities in preventive health behavior.41-46 Shokar et al47 reported that Whites and African Americans tended to differ in terms of how they valued the attributes of different CRC screening tests. Inadomi et al48 reported that among patients in an urban health care clinic who were offered SBT and colonoscopy screening, adherence varied by race and ethnicity.
Members of our research team previously reported on the use of decision staging, based on the Precaution Adoption Process Model (PAPM), to identify an individual's preferred CRC screening test.38,49 Findings from a recently reported study conducted by the group showed that aligning patient preference for a given CRC screening test with access to that test boosted screening adherence in a diverse primary care clinic patient population.50 Making both SBT and colonoscopy screening options available, offering choice related to these tests, and providing telephone navigation have been effective approaches to raising CRC screening rates, especially among minority patients.36,38-40,48-55
Regarding the matter of choice, a systematic review56 identified studies in which participant preference for CRC screening tests was assessed on the basis of test attributes and test delivery attributes. In the studies, CRC screening test preference was elicited using indirect approaches, including stated preference methods that presented hypothetical tradeoffs relative to different features of the tests, or revealed preference methods based on actual behavior or test purchases. None of the studies reported using preference assessments prospectively to guide intervention contacts intended to encourage screening adherence. In the current study, test preference is assessed prospectively to generate information used in a decision support and navigation intervention that is intended to facilitate completion of the participant's preferred screening test.
Decision Support and Navigation
Patient navigation is recognized as a promising way to increase adherence to recommended health behaviors. Traditionally, patient navigators have been nurses who are assigned to help patients diagnosed with cancer find their way through the complex and confusing diagnostic and treatment process.57-62 Trained health educators and lay health advocates have also acted as patient navigators to effectively facilitate preventive health behaviors.63 In terms of cancer screening, most navigation studies have addressed breast and cervical cancer screening; a small number of studies have focused on CRC screening.38,50,63 A few CRC screening studies have involved contacting the general primary care patient population residing in the community. In those studies, a patient navigator called patients by telephone and encouraged them to arrange colonoscopy screening. This strategy has generated screening rates ranging from 27% to 41%.36,49,53 A recently reported randomized trial50 determined that CRC screening adherence was significantly higher among African American primary care patients who were mailed CRC screening tests and received patient navigation outside of a practice visit vs patients who only were mailed the CRC screening tests (38% and 24%, respectively).
In several studies, primary care providers issued a colonoscopy screening referral for patients at the time of an office visit, and then “handed off” the patients to a navigator authorized to arrange a screening appointment. This approach produced screening rates ranging from 54% to 66%.64,65 Two systematic reviews66,67 reinforced the observation that patient navigation is an effective strategy that can raise CRC screening rates, especially in populations with limited English proficiency and those that have experienced disparities, and when the patient navigator assumes responsibility for appointment scheduling.68-70 For minority patients who have an office visit, Inadomi et al and others34,51 reported that providing patients with a choice of CRC screening tests along with patient navigation at the time of an office visit can result in high CRC screening rates among minority patients.
Research is needed on intervention strategies that seek to reach minority and underserved primary care patients who are not up to date with CRC screening guidelines and might not have had a recent office visit. Given findings from the studies cited, it would be useful to reach out to such patients in order to offer a choice of CRC screening tests, identify the CRC screening test that patients prefer, and provide support for performance of a preferred test.54,55 We designed the study described here to test the impact of this type of intervention among Hispanic primary care patients who were eligible for CRC screening.
Participation of Patients and Stakeholders
We formed a patient and stakeholder advisory committee (PASAC) as part of the proposal development process. The PASAC was composed of patients from the Lehigh Valley Health Network (LVHN) (not study participants), Hispanic community leaders, and LVHN providers and administrators. The patients described themselves as veterans, clergy, patient support staff, housewives, nurses, and community members. All committee members were bilingual. PASAC members were recruited from local community-based organizations, local churches, and LVHN primary care practices, representing the diverse “voices” of the Hispanic community in Lehigh Valley. The PASAC also included LVHN providers and administrators who were in positions to understand primary care CRC screening activities in the health system and were available to answer questions about how the project fit in the context of LVHN primary and specialty care. Regularly participating community members included 8 individuals—4 men and 4 women.
The PASAC met 3 times per year for the duration of the study in an LVHN hospital community conference room, including an initial meeting that was convened prior to implementation of study participant contact and intervention delivery. The meetings, which were co-led by Dr Brian Stello (principal investigator of the study site) and Ms Myra Piña (community member), were conducted in both English and Spanish. This committee's primary purpose was to review processes, procedures, and materials; comment on regular reports on study progress delivered by the academic research team; and provide feedback and recommendations in keeping with both PCORI and community-based participatory research principles of community and stakeholder involvement in research. With permission from the members, we audio-recorded each session. PASAC members made many important observations and recommendations related to study operations. Below, we highlight those that were particularly remarkable and helpful to the study.
- PASAC members informed the academic research team that while either Hispanic or Latino were terms that could be used to describe the ethnicity of community members, most individuals in the local population to be approached in the study would likely favor the term Hispanic. We included this terminology in all patient education materials used in the study.
- PASAC members suggested that print materials—those to be used for study recruitment as well as those to be used as part of the interventions—be presented in both English and Spanish. PASAC members reviewed all materials in both languages and helped frame terminology in plain language that was culturally sensitive and literacy appropriate. PASAC members reviewed and approved the pictures, text, and color scheme used to create print materials for the study.
- PASAC members advised the research team that the community would not understand the role of patient navigation in relation to primary care. They recommended using the term asistente del paciente when patient navigator contacts were made. The members also strongly advised that patient navigators be fluent in Spanish and be prepared to interact with study participants in their language of choice. The study's print materials used the asistente del paciente terminology when referring to the patient navigator, who was prepared to communicate with study participants in the language of their choice.
- PASAC members also recommended making financial counseling support available. Importantly, we made this service routinely available to study participants as part of a pre-colonoscopy visit that explained how to prepare for the procedure, address unanswered questions, and deal with unmet financial needs. We made available financial assistance to all patients at the time of an initial visit to prepare for screening colonoscopy.
- Furthermore, PASAC members played an instrumental role in conceiving of, advocating for, organizing, and attending an end-of-study community conference at a CRC screening health fair. We viewed this event as an important expression of appreciation to the Hispanic community for its support of the research study and as a vehicle for sharing study results. These and other observations and suggestions made by PASAC members helped shape patient education and intervention materials and methods, as well as contributed to the dissemination of study findings.
Methods
Prestudy Focus Groups
Prior to initiation of the randomized trial, we conducted 2 focus groups. We developed the focus group facilitator's guide with input from research teams from all 3 collaborating institutions: Thomas Jefferson University, LVHN, and the Fox Chase Cancer Center. We based the guide on current literature regarding barriers and facilitators to colorectal screening in general and specifically in Hispanic populations, and we provided the protocol for moderating the focus group.
The focus groups addressed perceptions of ethnicity and race, study recruitment strategies and procedures, and terms and approach for the navigator. Specifically, the study team wanted to understand how patient navigation was viewed in the Hispanic community. Additional attention was allotted to assessing the appropriateness of the drafted educational materials and decision support procedures to be used in the intervention. This assessment included cancer content, health literacy, and cultural appropriateness. The groups reviewed patient recruitment materials (ie, invitation letters and study description letters, as well as intervention materials, including an educational screening brochure). With each item reviewed, we elicited participants' perceptions about intervention contact and barriers to and facilitators of screening. Group moderators also solicited feedback on the study process issues; for example, asking if people would rather be contacted through a letter or phone call. The focus groups were audio recorded, transcribed, and translated.
LVHN staff recruited focus group participants by working with its network members and local community agencies to identify individuals for the focus groups. Inclusion criteria included individuals who self-reported being of Hispanic ethnicity, age 50 to 75 years, having an affiliation with a community group whose mission included attention to Latino community interests, and/or being a patient in the LVHN system. Focus group participants were representative of the targeted Hispanic population. Feedback from the focus groups helped the research team develop training sessions for the study research assistant and patient navigator that focused on dealing with patient concerns related to the following: screening-related costs, fear of a cancer diagnosis, worry about taking the prep needed to undergo screening colonoscopy, anxiety related to the invasiveness and safety of the colonoscopy procedure, feelings of machismo among male study participants, concerns about the need to take time off work to undergo screening colonoscopy, the challenge of contacting and following participants due to frequent changes in residence and telephone numbers, and the strong desire of community members to take care of family members' health-related issues before (instead of) their own preventive health care.
Participant Recruitment and Allocation to Study Group
At defined intervals during the study, we used LVHN electronic medical records to identify patients in 5 participating primary care practices who were potentially eligible for the study. Participating practices ranged in size from more than 900 active patients to nearly 8000 active patients. The practices included a Hispanic patient-focused internal medicine practice, a hospital-based internal medicine practice, a large family medicine practice staffed by residents and attendings, a small community-based family practice, and a Federally Qualified Health Center (FQHC) family medicine practice. Medical records review prior to patient recruitment suggested the CRC screening rate among age-eligible Hispanics in the practices was nearly 30%.
When we initiated the study, we defined eligible primary care practice patients as those who were identified as Hispanic in the electronic health record, were aged 50-75 years, had not been diagnosed with CRC, and had not had a CRC screening test according to guidelines (ie, an SBT within the previous 12 months or a colonoscopy within the previous 10 years). Once we identified potentially eligible patients, we assigned them to a total of 10 contact cohorts. We mailed individuals in each cohort a study invitation letter in English and Spanish. The letter described the research study and provided an opportunity for the recipient to opt out of participation.
A trained bilingual research assistant called patients about 15 days after the invitation letter mailing. The research assistant attempted calls throughout the work day, as well as during evenings and on weekends in order to reach a diverse working population, and conducted the call in the language preferred by the respondent. During the call, the research assistant verified patient eligibility, described the study, explained that participants would not be required to pay for SBT (though colonoscopy completion would be billed through normal methods), determined patient willingness to participate in the study, obtained verbal consent, and administered a brief baseline survey. The research assistant passed the names of study participants to a research coordinator for random assignment to a study group.
Note: In terms of eligibility for CRC screening, the research assistant excluded those individuals who self-reported being up to date with screening tests, including USPSTF and ACS CRC screening guidelines that were in place at the beginning of the study.
As in previous work by the research team,38,49 items on the survey described SBT as a test to check for CRC—done at home using a set of cards—and a colonoscopy as a test that examines the entire colon using a narrow lighted tube, which is inserted into the rectum (tests most commonly recommended for screening in primary care). Respondents were asked to report their PAPM71 screening decision stage for each test (1 = decided against, 2 = not considering, 3 = undecided, or 4 = decided to do).
We analyzed responses to the PAPM items to determine the individual's proximity to screening with any test (overall screening preference) and proximity to performance of a given test (screening test preference). For example, if a participant reported that he or she had decided to do colonoscopy screening and was undecided about doing SBT, we assigned the individual's overall screening preference as decided to do screening; colonoscopy was then assigned as the individuals' preferred screening test. Data on health literacy, preferred language, and sociodemographic background were also collected. At the end of the baseline survey, the interviewer confirmed contact information and asked for preferred day(s) and time(s) for future contacts. Finally, the research assistant informed the participants that they would receive CRC screening materials in the mail, explained that the research team might contact them by telephone to discuss screening, and mentioned that a team member would contact them to complete a follow-up survey. The research assistant was blinded to subsequent study activities related to participants.
The study's biostatistician was responsible for developing a computer-generated randomization sequence that stratified study group assignment by primary care practice (5 strata) and used blocking to assign participants to either a decision support and navigation intervention (DSNI) group or a standard intervention (SI) group in a 1:1 ratio. Using the generated randomization schedule, a study research coordinator, separate from the research assistant conducting recruitment and baseline survey activities, allocated assignments to enrolled participants on a weekly basis in the order they were consented. Following randomization, the research coordinator provided contact information for DSNI group participants to the study's patient navigator. All research staff members who conducted end point assessments (surveys and medical record reviews) were blinded to participant study group assignment.
Intervention Implementation
We mailed SI group participants a set of standard materials, which included a letter from the participant's primary care practice encouraging selection and performance of either (1) colonoscopy screening or (2) SBT screening; an informational brochure describing the CRC screening tests; instructions for arranging a screening colonoscopy appointment; and instructions for completing an immunochemical SBT. The SBT used was the Polymedco FOBT-CHEK (Fecal Occult Blood Test) 1-specimen test. We included instructions in English and Spanish. Specimen tubes were prelabeled with patient identifiers. Patients were asked to fill in the collection date on the specimen and enclose the tubes in their return envelope. Print materials were provided in both English and Spanish. At 45 days following random assignment, if no screening was completed, research staff sent participants a reminder letter that encouraged screening.
Study participants assigned to the DSNI group were mailed the same set of bilingual print materials. A bilingual patient navigator, hereafter referred to as patient assistant, attempted to reach DSNI participants within 7 days following mailing. During the call, which was delivered by 1 patient assistant to all DSNI participants, the patient assistant followed a script reviewing highlights of the CRC informational booklet and verified the participant's preferred CRC screening test. Following verification of preferred test, the patient assistant used an online Decision Counseling Program© (DCP) to identify barriers to and facilitators of preferred test performance and to determine the likelihood of test performance.
The online DCP included a script guiding the patient assistant and participant through a structured telephone encounter. Following the script, the patient assistant elicited major factors that would influence the participant to complete or not to complete (pros and cons) his or her preferred screening test, determined the level of importance the patient assigned to each factor, entered the reported factors and factor weights into the DCP, and computed a screening likelihood score (0-1). The patient assistant then reviewed the result with the patient. The patient assistant used reported barriers identified within the DCP and test specific procedures to develop a personal screening plan in conjunction with the participant.
We included the steps of each participant's screening plan in a 1-page summary, which we sent to the participant and to his or her primary care physician. As participating primary care practices used an electronic health record (EPIC), the patient assistant uploaded the participant's screening plan directly into the medical record and later contacted the practice office manager to verify its receipt. Following completion of the navigation call, patient assistant follow-up was dictated by the participant's screening plan and preferred screening test. For participants who preferred colonoscopy screening, the patient assistant obtained the appropriate referral. In addition, the patient assistant arranged a prescreening office visit to review preparation for the procedure and address any financial issues or concerns for those who were uninsured or underinsured. For participants who preferred SBT screening, the patient assistant reviewed the SBT kit and made a follow-up telephone call to participants to address emergent questions or concerns related to test completion. Similar to the SI group, at 45 days postrandomization, DSNI participants with no indication of screening received a mailed reminder. For study participants who underwent screening colonoscopy, test results were sent to the individual's primary care physician, who then reported findings to the individual. SBT results were sent directly to the patient and his or her primary care physician.
Six months following randomization, research study staff completed a medical record review for all participants. We then used data from the medical record review to create a Screening Status Report for both SI group and DSNI group participants. This report was uploaded into the electronic medical record and routed to the patient's primary care physician. The report indicated whether the participant had adhered to screening and if he or she was in need of diagnostic follow-up for an abnormal SBT result. For those participants who were nonadherent to CRC screening, the report advised providers to discuss screening with the patient. For patients who had a normal SBT result, the report encouraged providers to contact patients in a year to offer screening.
Additionally, 6 months postrandomization, a study survey interviewer contacted each participant by telephone and administered a survey. The survey included CRC screening decision stage items (described earlier) identical to those on the baseline survey. Additionally, the survey assessed participant knowledge about CRC screening based on a 10-item scale developed by the research team; religiosity and fatalism measured with 5 items assembled and adapted using 2 existing tools, the Powe Fatalism Inventory72 and the Religious Health Fatalism Questionnaire73; social support measured by the 8-item emotional/informational support subscale of the MOS Social Support Survey74; and CRC screening perceptions as measured by the Preventive Health Model (PHM).75 Specific PHM constructs included perceived CRC susceptibility, salience and coherence, worries and concerns, response efficacy, fatalism, acculturation, and social support for screening.
Finally, 12 months postrandomization, we performed an end point medical records review on all study participants to assess CRC screening adherence. A study research assistant who was blinded to each participant's study group assignment performed the medical records review. Any CRC screening test that conformed to the guidelines recommended by the ACS or USPSTF was noted.
Poststudy Focus Groups
Following completion of the randomized trial, focus groups were conducted with study participants randomized to the DSNI group. The patient assistant at Lehigh Valley Hospital conducted recruitment for the focus groups. The first focus group included study participants who had screened following the intervention. This focus group occurred on the evening of January 10, 2017. The second focus group consisted of participants who had not screened. The second focus group took place on January 11, 2017. Both focus groups took place at the Lehigh Valley Hospital in Allentown, Pennsylvania. Both sessions were conducted mainly in Spanish with limited English; each session was audio-recorded with permission from all participants.
Each participant in the focus groups received a $50 gift card for his or her time and travel. The primary focus of the discussion revolved around what participants thought about the study intervention and their reasoning for screening or not screening. Prior to beginning the session, the study team reviewed the informed consent and obtained approval from all participants.
Dissemination
At the direction of the PASAC, the research team convened a CRC Screening community conference on March 25, 2017, in the PPL Center and Arena Concourse in Allentown. The research team and PASAC members provided information on CRC screening; findings from the randomized trial; an opportunity to tour an inflatable colon; and a simulated demonstration of a colonoscopy procedure utilizing an endoscope, a model colon, and a video monitor. More than 30 LVHN and community organization exhibitors also offered information and services related to a variety of health topics.
Data Management
Data sources included a main tracking database (Access), baseline survey and intervention activities data file (REDCap), navigation logistics database (Excel), DCP data file (Excel), end point medical records review data file (REDCap), and end point survey data file (Excel). For analysis purposes, data were merged across these databases using a unique study identifier. Study identifiers were assigned at the beginning of each successive enrollment cohort. Prior to analysis, we used SAS programs to facilitate data merging, checking, and validation. We have used this system successfully in multiple prior studies and have extensive code for checking data validity and consistency (within and across fields/variables).
Study End Points
The primary study end point was overall screening adherence within 12 months of the randomization date by performance of any recommended CRC screening test. A secondary study end point was test-specific screening adherence via SBT or colonoscopy, the 2 most commonly recommended CRC screening modalities. We determined screening status through a medical records review performed at 12 months after randomization. This was supplemented by information provided on the end point survey conducted 6 months after randomization, which we used to capture tests that might have occurred outside the system and not reflected in the medical records (in practice, this occurred only for 4 participants). Secondary study end points were the baseline-to–end point change in CRC screening decision stage, the end point score on perceptions regarding CRC and screening, and the end point score on knowledge regarding CRC and screening (all measured via self-report on the participant surveys and described previously).
Data Analyses
CRC Screening Adherence
We analyzed overall screening adherence (dichotomous screening end point: any vs none) via logistic regression. The prespecified analysis plan in the protocol defined that the following variables would be included in the main model: study group, practice, gender, age, and any baseline characteristic that appeared to be different across the 2 study groups. We included practice because it was a stratification factor during randomization, while we included gender and age to allow for additional testing of interaction terms with the study group in secondary analyses.
Upon analyzing the data, we found a difference between the SI and DSNI groups with respect to the preferred screening test at baseline; therefore, we also included preferred screening test and overall screening decision stage in the main model. We analyzed test-specific screening adherence (SBT, colonoscopy, or no screening) via multinomial (polytomous) logistic regression, with the same variables in the model as for overall screening adherence.
We also conducted analyses using the generalized estimating equations approach, using the robust variance in the logistic regression models to account for potential within-practice clustering. The results were practically identical with or without accounting for clustering, so only the latter are presented in this report.
All the main analyses followed the intention-to-treat principle. However, we also conducted as-treated analyses to explore subgroups of DSNI participants according to the degree of navigation they received (ie, no contact for navigation achieved, contact achieved and navigation performed, contact achieved but navigation not performed because participant had already screened or scheduled screening).
Overall Screening Decision Stage
We assessed overall screening decision stage at the baseline and 6-month participant surveys. Because there were only a few “backward” changes (from higher to lower decision stage), we combined them with “no change” and analyzed any “forward” change (from lower to higher decision stage) vs “backward or no change” via logistic regression.
Perceptions and Knowledge
We analyzed the baseline-to–end point change in scale scores (perceptions and knowledge regarding CRC and screening) via linear regression. We obtained the information on these secondary end points through the participant surveys. Corresponding analyses included all baseline covariates to control for imbalances between the 2 groups that might be introduced by potentially differential nonresponse patterns.
Process Measures Related to DSNI Delivery
We summarized descriptively operational data on navigation contacts (eg, number and timing of navigator call attempts, time from randomization to initiation and completion of contact, validation of test preference, reasons for incomplete navigation call delivery, and details related to delivery of the mailed screening plan and screening status report).
Patterns of Screening Adherence Beyond 12 Months
Using electronic health records data for participants who completed an SBT, we determined subsequent patterns of screening beyond the study's 12-month observation period. For individuals with a negative index SBT, guidelines recommended another SBT or a colonoscopy 12 months later; therefore, we summarized the rate of rescreening within 15 months (ie, 12 months plus a 3-month grace period). For individuals with a positive index SBT, we summarized rates of a subsequent diagnostic colonoscopy.
Intervention Reach, Effectiveness, Adoption, Implementation, and Maintenance Measures
Finally, we assessed intervention reach at the patient level in terms of the number/percentage of study participants who received planned intervention contacts, compared with those who were eligible to receive such contacts. We also determined the number/percentage of providers that received participant screening plans and reports. In addition, we asked navigators about their experiences in delivering intervention contacts to participants and providers. We used mail and telephone records, along with navigator logs, to obtain data to measure this dimension.
We measured intervention efficacy/effectiveness at the patient level in terms of impact on defined primary outcomes (ie, screening adherence, screening decision stage, and test-specific adherence). Here, we used data from the 6-month survey and the 6- and 12-month medical record reviews to evaluate this dimension. In terms of intervention adoption, we examined the participation of invited practices and their characteristics.
We assessed intervention implementation by determining the number and timing of attempted and completed navigator calls, time from call initiation to call completion, length of calls, and reasons for incomplete calls. In addition, we collected qualitative data from the navigators about their experience implementing the intervention: what worked well and what did not, whether patients utilized their assistance, and which key services might have contributed to completion of screening. Finally, we interviewed select health system leaders to gain insights that would help us to form impressions about the potential for DSNI maintenance in the health system.
Sample Size and Power
We designed the study with the assumption that 12-month screening rates would be 45% in the DSNI group (30% SBT plus 15% colonoscopy) vs 30% in the SI group (20% SBT plus 10% colonoscopy). With these assumptions, and using a 2-sided ɑ of .05, the target sample size of 400 had 85% power for overall screening adherence and 80% power for test-specific screening adherence (with an allowance for up to 5% missing data for the primary end point built into the calculations).
Results
The research team queried LVHN administrative data at 2 points in time to identify a total of 2720 Hispanic patients who were potentially eligible for CRC screening and sent invitation letters. Figure 1 shows that we attempted to contact 2622 of these individuals by telephone to assess study eligibility and interest (3 patients were ineligible prior to contact and contact attempts were not initiated with 95 patients because we had achieved the study recruitment goal). We were unable to reach 1280 patients (563 were found to have a wrong telephone number and we discontinued contact efforts for 717 patients after multiple calls were made and messages were left). We determined that 686 were ineligible for screening (504 self-reported being up to date with screening and 182 did not meet other eligibility criteria) and we contacted 256 patients that refused to participate in the study.
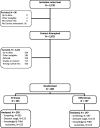
Figure 1
CONSORT Diagram.
Four hundred patients were consented, surveyed, and enrolled in the study. The participation rate, based on the original sampling frame of potentially eligible patients for whom contacts were attempted outside the context of an office visit, was approximately 15%. This rate is actually higher than that achieved in prior studies, in which contacts were attempted with patients outside the context of an office visit.38,50 We randomly assigned all consented patients to 1 of the 2 study groups, with 203 (51%) assigned to the SI group and 197 (49%) to the DSNI group. We administered an end point survey 6 months postrandomization (median, 210 days; interquartile range, 195-230 days). Of the 400 participants, 253 (127 SI, 126 DSNI) responded to the 6-month survey. We performed a 12-month end point medical records review for all randomized participants.
Table 1 shows that participant characteristics were as follows: female (59%), aged 50- to years (69%), less than or equal to a high school education (73%), and unmarried (54%). In addition, 71% of participants reported having an annual family income of less than $15 000 and 71% noted they had some form of insurance. Furthermore, 72% of participants said they had lived in the United States for >10 years; 80% indicated Spanish was the most common language spoken at home. The 2 study groups were comparable regarding most participant baseline characteristics, with the exception of preferred screening test (with SBT being the preferred test for a higher proportion of SI group than DSNI group participants).
Table 1
Patient Characteristics.
In secondary analyses, we compared study participants with individuals in the study sampling frame. Results of these analyses showed LVHN patients from the AIDS Activity Office (AAO) and Centro de Salud (CDS) primary care practices and patients aged 60 years or younger were overrepresented among participants (P = .044 and P = .001, respectively).
CRC Screening Adherence
Table 2 summarizes the results of the intention-to-treat analyses of the screening outcomes. At 12 months, CRC screening was substantially higher in the DSNI group than in the SI group (78% vs 43%), with an adjusted odds ratio (OR) of 4.83 (95% CI, 3.08-7.58; P = .001). Compared with the SI group, the DSNI group was more likely to undergo both SBT (57% vs 37%; OR, 4.20; P = .001) and colonoscopy (20% vs 6%; OR, 8.79; P = .001). The complete model results are presented in the Table 2 supplement. The results were very similar for the 6-month screening rates.
Table 2
Overall and Test-specific Screening Adherence.
Table 2 Supplement
Full Model Results of Overall Screening Adherence.
In secondary as-treated analyses, we subdivided the DSNI group according to the contacts that participants actually received and compared them to the SI group (43% screened at 12 months) as a reference. We successfully contacted 166 (84%) of the 197 DSNI participants who were targeted to receive the intervention. Thirty-eight of these DSNI patients contacted for navigation reported that they had already been screened or had scheduled screening at the time of the navigation attempt. At 12 months, 37 (97%) of these participants had screened (37 of 38; OR vs SI group = 50.7; P = .001), while those who were contacted and underwent navigation had 83% screening (106 of 128; OR vs SI group = 6.74; P = .001). In contrast, participants who the study was unable to reach to deliver navigation had only a 32% screening rate (10 of 31; OR vs SI group = 0.66; P = .316).
There was no discernible interaction between study group and gender: The adjusted OR for DSNI group vs SI group was 5.44 among women and 4.14 among men (interaction P = .549). The intervention effect appeared somewhat stronger among younger participants, with an adjusted OR of 5.67 among participants 60 or younger and 3.41 among participants 60 and older, although the interaction between study group and age was not statistically significant (P = .292).
Although randomization achieved good baseline comparability of the SI and DSNI groups, we expanded the main analyses to include all baseline characteristics in order to identify additional predictors of screening. In these analyses, study group remained as a strong, significant predictor of adherence (OR, 5.07; 95% CI, 3.13-8.21; P = .001). Additionally, participants who had been living in the United States for less than 10 years had 3.5-fold higher odds of screening compared with individuals who had been living in the United States for 10 years or longer or to native-born residents (OR, 3.49; 95% CI, 1.71-7.10; P = .001). The odds of screening for those with baseline decision stage of “decided to do” were double that of participants who had not “decided to do” (OR, 1.99; 95% CI, 1.03-3.84; P = .040). The remaining factors were not significantly associated with screening adherence.
Finally, we sought to determine repeat screening (beyond the formal end of the study observation period) for participants who elected to screen via SBT. In both groups, 189 participants screened via SBT, and they would again be eligible for screening 12 months later (plus a 3-month grace period). For 162 of those 189 participants, enough time (15 months) had elapsed between their index SBT date and the end of the study (March 2017). Therefore, we conducted an additional medical records review to determine whether they had kept up with their screening, even though they did not receive any study interventions. Of the 153 participants with a negative index SBT result, 40 rescreened within 15 months (SI: 19 of 57 = 33%; DSNI: 21 of 96 = 22%; P = .119). SBT was the most common rescreening modality (58% in SI, 62% in DSNI). Finally, 4 of the 9 participants with a positive index SBT result underwent a diagnostic colonoscopy (SI group: 1 of 2; DSNI group: 3 of 7).
Overall Screening Decision Stage
At the baseline survey, the 2 groups were comparable regarding overall screening decision stage (Table 1). At the end point survey, most participants in both groups reported a decision stage of “screened” or “decided to do.” Table 3 summarizes baseline and end point decision stages for participants in the 2 study groups, as well as the baseline-to–end point changes. Overall, 97 (79%) DSNI group participants reported a forward change in decision stage compared with 61 (50%) SI group participants (adjusted OR, 4.91; 95% CI, 2.55-9.47; P = .001).
Table 3
Screening Decision Stage.
CRC Screening Perceptions and Knowledge
Table 4 summarizes the results for the CRC screening knowledge and perceptions measured for participants in both study groups on the 6-month survey. Perceptions were very similar between the 2 groups. The average overall PHM score was 3.8 for both DSNI and SI group participants (adjusted mean difference for DSNI vs SI = −0.01; 95% CI, −0.15 to 0.13; P = .862). The DSNI and SI groups were not significantly different on any of the subscales. Similarly, the 2 study groups were very similar on the knowledge test, with an average correct responses of 4.6 in the DSNI group vs 4.7 in the SI group (adjusted mean difference for DSNI vs SI = 0.04; 95% CI, −0.47 to 0.55; P = .880).
Table 4
CRC and Screening Perceptions and Knowledge.
Additional Analyses of Decision Support and Navigation Process
Of 197 DSNI group participants, 166 (84%) were reached for navigation. Of those, 27 had already screened and 11 had already scheduled a colonoscopy. The remaining 128 (65%) were navigated. For the 166 participants who were reached, their preferred test and overall screening decision stage were reassessed. Of the 27 who preferred SBT at baseline, 93% continued to prefer SBT, while 41% of the 17 with an initial preference for colonoscopy continued to prefer colonoscopy. Of the 122 with an equal preference for the 2 screening tests at baseline, 65% expressed a preference for SBT screening at the time of navigation and 35% preferred colonoscopy screening.
Poststudy Focus Groups
Of the randomized trial participants invited to participate in the focus groups, 6 individuals attended the “screeners” focus group and 6 attended the “nonscreeners” meeting. All of the participants were bilingual and both groups comprised 3 women and 3 men. Focus group interactions were audio-recorded, transcribed, and content analyzed. The interactions allowed for information sharing and decision support. It was evident from reported interactions with the patient navigator that the participants felt very comfortable with that research team member. Most participants were pleased with the information provided and that it was translated into Spanish. The study influenced both groups, increasing their awareness of the CRC burden, screening guidelines, and options. Many mentioned the notion of “prevention” as a motivating message. Most were willing and eager to share information with others. However, a few remained resistant to screening although they recognized the importance. The focus groups provided insights into the challenges patients face even when their interest and motivation have increased and that system issues (such as transportation to appointments, calling with results, and opportunities to do the SBT at the provider's office) should be considered. In addition, the initial mailing and subsequent contact with the patient navigator were viewed as important in the process of CRC screening.
Reach, Effectiveness, Adoption, Implementation, and Maintenance Analysis
Reach
In order to enroll 400 participants, we used administrative data sources to select 3 cohorts of potentially eligible patients who were targeted for recruitment (cohort 1: 2345, cohort 2: 275, and cohort 3: 437). We assessed reach based on the selection of participants enrolled in the first cohort of patients. The sampling frame for this analysis included 2345 potential participants from the initial cohort. According to medical records, these patients were Hispanic in terms of ethnicity, were aged 50-75 years, had no personal or family history of CRC and polyps, and were not up to date with CRC screening. We mailed all individuals in the sampling frame a study invitation letter and attempted to contact them by telephone to facilitate recruitment into the study. A total of 333 (14%) individuals from the sampling frame satisfied eligibility criteria, were contacted by telephone, consented to participate in the study, and completed a baseline survey.
Effectiveness
(Described under Results: CRC Screening Adherence)
Adoption
Five primary care practices affiliated with LVHN were invited to participate in this research study; all (100%) agreed to participate. The 5 practices served approximately 15 792 LVHN patients. In terms of practice staffing, the practices employed 26 physicians, 67 residents, 17 RNs, 8 NPs, 1 PA, and 49 other support staff. Only 1 practice had a patient navigator on staff.
Implementation
On average, 4.1 (SD, 2.8) calls were required to complete navigation (range, 1-11 calls). We mailed the screening intervention kit at an average of 6.4 (SD, 5.2) days postrandomization (range, 0-28 days), and the first navigation call attempt was made 14.3 (SD, 8.1) days after randomization (range, 2-65 days). We discontinued navigation contacts at an average 29.3 (SD, 20.2) days postrandomization (range, 3-139). Individual interviews with the research assistant and with the patient navigator were conducted. Interviews were recorded and were transcribed. Three members of the research team transcribed content. Coders met to reconcile differences in coding and to identify thematic categories represented in the interview data. We identified the following 4 themes in the interview data through content analysis of the research assistant's and patient navigator's perceptions: (1) how well their training prepared them to interact with patients and the effectiveness of their patient intervention contacts, (2) adjustments they had to make to provide study-related information to participants and deliver intervention contacts, (3) barriers to intervention implementation, and (4) intervention effects on study participants and the Hispanic community.
Maintenance
We conducted 30-minute interviews with leaders in the practices in which the decision support intervention was implemented in order to understand their experience of the intervention and to probe its potential continuation after completion of the research. Three leaders have been interviewed to date: a medical director, community outreach coordinator, and care manager–office coordinator at different sites. All informants identified positive benefits of the intervention: A bilingual navigator addressed language, literacy, and cultural sensitivity needs; navigator involvement facilitated work flow; and DSNI enabled practice to achieve quality goals. Some barriers to intervention maintenance include providing administrative support for navigation, dedicating a patient navigator to focus on the task of boosting CRC screening rates, and determining how to pay for colonoscopy screening for patients who are underinsured or uninsured. Nonetheless, respondents expressed enthusiasm for the incorporation of DSNI into the health systems as a centralized approach that could help practices and patients.
Discussion
Decisional Context
This research has addressed an important evidence gap in a sustained research program on the implementation of evidence-based CRC screening interventions. As described earlier, many studies have shown that patient navigation can increase CRC screening rates among primary care patients of different racial/ethnic background who are not up to date with CRC screening guidelines, are recruited into a study during a visit to a primary care practice, and begin the navigation process at the office visit. Few trials have been conducted with nonadherent patients who are recruited into a study, are given access to SBT and colonoscopy screening tests, and receive decision support and navigation contacts outside the context of an office visit. To our knowledge, this study is unique in testing DSNI in a health system among Hispanic primary care patients.
Given findings reported in the literature and those from our own research, we reasonably believe that giving patients access to both SBT and colonoscopy screening options, helping them decide on a preferred test they are likely to do, and navigating them through the performance of their preferred test can have a positive impact on screening rates. The magnitude of this effect was substantial among Hispanic patients in this study. It is possible that the application of these methods by health systems could help reduce CRC screening disparities between Hispanic and non-Hispanic Whites. However, additional research is needed to demonstrate intervention impact on this disparity.
Unfortunately, the centralized implementation of evidence-based CRC screening interventions, such as DSNI, has been limited in health care systems. As a result, we continue to observe screening disparities in primary care subpopulations, including African American and Hispanic patients.76-78 Going forward, we need implementation science research on how to move DSNI and other proven cancer screening interventions into routine care.79,80 Moreover, health systems and insurers should develop collaborative models of care that support the implementation of evidence-based interventions for those they serve.
Study Results
We discovered that CRC screening adherence was significantly and substantially higher in the DSNI group compared with the SI group. While this result was consistent with the hypothesized impact of exposure to the combined mailed material as well as decision support and navigation contacts vs mailed materials only, the magnitude of the DSNI effect is remarkable. While high rates of CRC screening in response to patient navigation have been reported elsewhere,64,65 those reports focused on the initiation of patient navigation with patients at the time of a primary care office visit. Here, decision support and navigation contacts were delivered to patients outside the context of a primary care office visit. Furthermore, no other studies have reported a comparable impact on CRC screening in Hispanic/Latino primary care patient population.
Overall, we discovered that when provided both colonoscopy screening and SBT screening alternatives, most study participants who adhered to screening did so by completing SBT screening. This finding is not surprising, as this test is more readily accessible because it is less expensive and more convenient than colonoscopy screening. We also observed that when given information about both SBT and colonoscopy screening alternatives, access to both tests, and the opportunity to discuss the pros and cons of screening with a patient assistant, most study participants preferred SBT screening over colonoscopy screening—and followed through on that expressed preference. We believe this finding is novel.
Another important finding from this study is that the DSNI group had significantly higher odds of screening via colonoscopy compared with the SI group and much higher odds of screening via SBT compared with their SI group counterparts. The literature includes several reports showing that exposure to different intervention approaches have increased colonoscopy screening rates or SBT screening rates. To our knowledge, however, this study is the first to report that both colonoscopy and SBT screening rates increased because of deploying an intervention designed to boost CRC screening rates. Implementation science research is needed to advance our understanding of how to increase the use of both screening tests in health systems.
Importantly, the increased SBT screening rate achieved because of exposure to DSNI was not maintained in the absence of subsequent intervention contacts. In fact, the SI group had a higher rate of rescreening than the DSNI group, although this difference was not statistically significant. This important finding is likely due to the fact that the SI patients who screened initially did so through minimal intervention without the benefit of a navigator. It is reasonable to believe that this group of participants represented a “self-motivated” group of patients. The DSNI screeners, on the other hand, included both self-motivated individuals and patients who required more assistance. This finding speaks to the capacity of DSNI to reach patients who would not have screened otherwise. This group, most of whom would have had a normal SBT result, probably required some form of notification or supportive contact to encourage repeat screening. It is also notable that most patients with an abnormal SBT result had not undergone colonoscopy at the time of the 6-month chart audit. This finding highlights the need to ensure the performance of complete diagnostic evaluation as part of a comprehensive CRC screening program.
We did not observe statistically significant differences in participant knowledge and perceptions related to CRC screening at 6 months. Given that the study groups were comparable in terms of sociodemographic background and most other characteristics, we assume that the groups were comparable in terms of CRC screening knowledge and perceptions at baseline. The lack of differences between the study groups, coupled with the substantial effect of DSNI on screening adherence, test-specific adherence, and decision stage, is notable. All participants received the same mailed educational materials; therefore, it is perhaps unsurprising that DSNI and SI had comparable effects on participant CRC screening knowledge and perceptions. The DSNI group additionally had the opportunity to review the materials with the patient navigator. It is possible that DSNI had temporary impact at intervention but that the effect(s) diminished with time. Further research is needed to assess the nature and durability of DSNI contact effects relative to factors that motivate patient adherence.
Generalizability
The generalizability of findings reported may be limited, given that study participants were drawn from only 5 primary care practices in 1 health system. In addition, the Hispanic/Latino patient population served by participating practices and the health system may differ from the Hispanic/Latino patient populations (and subpopulations) that receive care in different health systems. However, Reach, Effectiveness, Adoption, Implementation, and Maintenance evaluation has noted the enrolled population is similar to that of the eligible but not enrolled population. Implementation across more practices within the LVHN system and integration with their current care coordinators will provide more evidence of generalizability within other health systems.
Subpopulation Considerations
In this study, we found that screening was not likely among DSNI participants who could not be reached by telephone and that the DSNI was less effective among DSNI participants who had lived in the United States for >10 years. Innovative approaches may be needed to boost screening in these subgroups.
Study Limitations
A singularly talented navigator who had undergone standard navigation and decision counseling training delivered the DSNI. Given that the intervention was delivered by 1 female navigator, generalizability of results may be limited as we cannot account for differences related to gender and style of navigation that may exist in a multi-navigator program. To examine delivery variation and differences among genders, multiple navigators will be used for intervention delivery in future research. It should also be noted that we conducted the study with Hispanic patients who were identified in 5 primary care practices in 1 health system in eastern Pennsylvania. Unique characteristics of these practices (especially of practices that contributed a relatively large proportion of study participants), the organizational structure of the health system and its approach to CRC screening, and the particular sociodemographic and cultural background characteristics (eg, the high proportion of study participants who were aged 60 years and younger) of study participants may also limit the generalizability of study findings. Moreover, study participants consented to participate in a randomized trial, thus representing a self-selected population that might have been more responsive to study interventions than the patients in the larger Hispanic primary practice patient population. As a result, the magnitude of effects might be greater than those that would be observed in the general Hispanic primary care patient population.
Finally, it is important to note that the 6-month survey nonresponse rate was higher than anticipated (37% instead of 20%), reducing our power to detect differences between the groups related to decision stage, knowledge, and attitudes. This nonresponse rate was almost identical in the 2 study groups (39% for the SI group and 38% for the DSNI group). Furthermore, for those participants with end point surveys, baseline characteristics were similar for the SI and DSNI groups, suggesting that the nonresponse biases and mechanisms were similar in the 2 groups and that baseline comparability was not severely compromised. We cannot rule out nonresponse biases linked to unmeasured participant characteristics, however, and the direction of such biases, if they exist, is unclear.
Future Research
Moving forward, the research team aims to conduct a project that builds on findings presented within this report. The project team will engage a learning community of patients, providers, and other stakeholders to adapt and implement DSNI in a representative set of LVHN primary care practices and primary care patients who are eligible for CRC screening. This learning community will be engaged in implementing DSNI in the LVHN. We also hope to take advantage of EPIC, which is shared by all LVHN primary practices, to facilitate intervention delivery initially in study practices and, ultimately, across the LVHN and other health systems.
Conclusions
This randomized trial engaged patients and stakeholders in the process of developing and implementing DSNI in a health system to encourage CRC screening among Hispanic primary care patients. Findings from the trial indicate that exposure to DSNI, compared with SI, had a substantial and statistically significant positive impact on overall patient adherence and on the forward movement of patients (ie, the progression from being ready to screen to actually screening). DSNI also promoted the completion of both SBT and colonoscopy screening. Further research is needed to determine the effects of DSNI delivery in health care systems in the general patient population, for which screening rates are suboptimal; among Hispanic patients from areas of origin that differ from those included in the current study; and among other racial/ethnic patient populations that experience CRC screening disparities. Research is also needed to identify the independent effects of DSNI components, including making different recommended screening tests readily available and accessible to patients, clarifying screening test preference and likelihood of preferred test performance, navigating patients through performance of their preferred test, and delivering a patient CRC screening status report to primary care providers. Moreover, we need implementation science research to determine how to maximize intervention reach and effectiveness in “real-world” settings outside the context of a randomized trial.
References
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627-637. 188838716. [PubMed: 18838716]
- American Cancer Society recommendations for colorectal cancer early detection. Accessed July 17, 2017. https://www
.cancer.org /cancer/colon-rectal-cancer /detection-diagnosis-staging /acs-recommendations .html - Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians. Recommendations and practices, 2006-2007. Am J Prev Med. 2009;37(1):8-16. [PMC free article: PMC2727732] [PubMed: 19442479]
- Bandi P, Barrera E, Graves K, et al. Cancer facts and figures for Hispanics/Latinos, 2012-2014. Accessed November 13, 2017. https://www
.cancer.org /content/dam/cancer-org /research/cancer-facts-and-statistics /cancer-facts-and-figures-for-hispanics-and-latinos /cancer-facts-and-figures-for-hispanics-and-latinos-2012-2014.pdf - Gonzales M, Qeadan F, Mishra SI, Rajput A, Hoffman RM. Racial–ethnic disparities in late-stage colorectal cancer among Hispanics and non-Hispanic Whites of New Mexico. Hisp Health Care Int. 2017;15(4):180-188. [PMC free article: PMC6211799] [PubMed: 29237342]
- Barzi A, Yang D, Mostofizadeh S, Lenz H-J. Trends in colorectal cancer mortality in Hispanics: a SEER analysis. Oncotarget. 2017;8(65):108771-108777. [PMC free article: PMC5752479] [PubMed: 29312566]
- Pollack LA, Blackman DK, Wilson KM, Seeff LC, Nadel MR. Colorectal cancer test use among Hispanic and non-Hispanic US populations. Prev Chronic Dis. 2006;3(2):A50. [PMC free article: PMC1563962] [PubMed: 16539791]
- Shih YCT, Zhao L, Elting LS. Does medicare coverage of colonoscopy reduce racial/ethnic disparities in cancer screening among the elderly? Health Aff (Millwood). 2006;25(4):1153-1162. [PubMed: 16835198]
- Iglehart JK. Prioritizing comparative-effectiveness research—IOM recommendations (perspective). N Engl J Med. 2009;361(4):325-328. [PubMed: 19567828]
- Agency for Healthcare Research and Quality. NOT-HS-11-014: Special Emphasis Notice (SEN): AHRQ announces interest in priority populations research. Accessed November 13, 2017. https://grants
.nih.gov /grants/guide/notice-files /NOT-HS-11-014.html - Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544-573. [PMC free article: PMC3619726] [PubMed: 19998273]
- Meester RGS, Doubeni CA, Lansdorp-Vogelaar I, et al. Colorectal cancer deaths attributable to nonuse of screening in the United States HHS Public Access. Ann Epidemiol. 2015;25(3):2018-213. [PMC free article: PMC4554530] [PubMed: 25721748]
- Bradley CJ, Lansdorp-Vogelaar I, Yabroff KR, et al. Productivity savings from colorectal cancer prevention and control strategies. Am J Prev Med. 2011;41(2):e5-e14. doi:10.1016/j.amepre.2011.04.008 [PMC free article: PMC3139918] [PubMed: 21767717] [CrossRef]
- Office of Disease Prevention and Health Promotion. Cancer | Healthy People 2020. Accessed November 14, 2017. https://www
.healthypeople .gov/2020/topics-objectives /topic/cancer - Ramirez AG. Redes en Accion: the National Hispanic/Latino Cancer Network. Latino Cancer Report: Summary Recommendations for a National Hispanic/Latino Cancer Control Agenda. Baylor College of Medicine; 2014.
- Robie L, Alexandru D, Bota DA. The use of patient navigators to improve cancer care for Hispanic patients. Clinical Medicine Insights. Oncology. 2011;5:1-7. [PMC free article: PMC3040076] [PubMed: 21339858]
- Gonzalez SA, Ziebarth TH, Wang J, Noor AB, Springer DL. Interventions promoting colorectal cancer screening in the Hispanic population: a review of the literature. J Nurs Scholarsh. 2012;44(4):332-340. [PubMed: 23078262]
- Naylor K, Ward J, Polite BN. Interventions to improve care related to colorectal cancer among racial and ethnic minorities: a systematic review. J Gen Intern Med. 2012;27(8):1033-1046. [PMC free article: PMC3403155] [PubMed: 22798214]
- Stone EG, Morton SC, Hulscher ME, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med. 2002;136(9):641-651. [PubMed: 11992299]
- Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152(10):668-676. [PubMed: 20388703]
- Rawl SM, Menon U, Burness A, Breslau ES. Interventions to promote colorectal cancer screening: an integrative review. Nurs Outlook. 2012;60(4):172-181. [PMC free article: PMC3366042] [PubMed: 22261002]
- Kreuter MW, Clark EM, Oswald DL, Bull FC. Understanding how people process health information: a comparison of tailored and nontailored weight-loss materials. Health Psychol. 1999;18(5):487-494. [PubMed: 10519465]
- Rakowski W, Ehrich B, Goldstein MG, et al. Increasing mammography among women aged 40-74 by use of a stage-matched, tailored intervention. Prev Med. 1998;27(5):748-756. [PubMed: 9808807]
- Skinner CS, Strecher VJ, Hospers H. Physicians' recommendations for mammography: do tailored messages make a difference? Am J Public Health. 1994;84(1):43-49. [PMC free article: PMC1614921] [PubMed: 8279610]
- Rimer BK, Kreuter MW. Advancing tailored health communication: a persuasion and message effects perspective. J Commun. 2006;56(suppl 1):S184-S201.
- Kreuter MW, Sugg-Skinner C, Holt CL, et al. Cultural tailoring for mammography and fruit and vegetable intake among low-income African-American women in urban public health centers. Prev Med. 2005;41(1):53-62. [PubMed: 15916993]
- Strecher VJ, Kreuter M, Den Boer DJ, Kobrin S, Hospers HJ, Skinner CS. The effects of computer-tailored smoking cessation messages in family practice settings. J Fam Pract. 1994;39(3):262-270. [PubMed: 8077905]
- Brug J, Steenhuis I, Van Assema P, De Vries H. The impact of a computer-tailored nutrition intervention. Prev Med. 1996;25(3):236-242. [PubMed: 8781000]
- Kreuter MW, Strecher VJ. Do tailored behavior change messages enhance the effectiveness of health risk appraisal? Results from a randomized trial. Health Educ Res. 1996;11(1):97-105. [PubMed: 10160231]
- Bull FC, Kreuter MW, Scharff DP. Effects of tailored, personalized, and general health messages on physical activity. Patient Educ Couns. 1999;36(2):181-192. [PubMed: 10223022]
- Kreuter MW. Integrating culture into health information for African American women. Am Behav Sci. 2006;49(6):794-811.
- Kreuter MW, Skinner CS, Steger-May K, et al. Responses to behaviorally vs culturally tailored cancer communication among African American women. Am J Health Behav. 2004;28(3):195-207. [PubMed: 15152880]
- Powe BD. Promoting fecal occult blood testing in rural African American women. Cancer Pract. 2002;10(3):139-146. [PubMed: 11972568]
- Powe BD, Ntekop E, Barron M. An intervention study to increase colorectal cancer knowledge and screening among community elders. Public Health Nurs. 2004;21(5):435-442. [PubMed: 15363024]
- Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110(9):2083-2091. [PubMed: 17893869]
- Percac-Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009;24(2):211-217. [PMC free article: PMC2628981] [PubMed: 19067085]
- Lipkus IM, Skinner CS, Dement J, et al. Increasing colorectal cancer screening among individuals in the carpentry trade: test of risk communication interventions. Prev Med. 2005;40(5):489-501. [PubMed: 15749130]
- Myers RE, Bittner-Fagan H, Daskalakis C, et al. A randomized controlled trial of a tailored navigation and a standard intervention in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2013;22(1):109-117. [PMC free article: PMC5537598] [PubMed: 23118143]
- Wolf RL, Basch CE, Brouse CH, Shmukler C, Shea S. Patient preferences and adherence to colorectal cancer screening in an urban population. Am J Public Health. 2006;96(5):809-811. [PMC free article: PMC1470583] [PubMed: 16571715]
- Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care. 2008;46(Suppl 1):S10-S61. [PubMed: 18725820]
- Tilley BC, Vernon SW, Myers R, et al. The next step trial: impact of a worksite colorectal cancer screening promotion program. Prev Med. 1999;28(3):276-283. [PubMed: 10072746]
- Pignone M, Bucholtz D, Harris R. Patient preferences for colon cancer screening. J Gen Intern Med. 1999;14(7):432-437. [PMC free article: PMC1496606] [PubMed: 10417601]
- Sheikh RA, Kapre S, Calof OM, Ward C, Raina A. Screening preferences for colorectal cancer: a patient demographic study. South Med J. 2004;97(3):224-230. [PubMed: 15043327]
- Dolan NC, Ferreira MR, Fitzgibbon ML, et al. Colorectal cancer screening among African-American and White male veterans. Am J Prev Med. 2005;28(5):479-482. [PubMed: 15894152]
- Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(2):132-141. [PubMed: 12118972]
- Nelson RL, Schwartz A. A survey of individual preference for colorectal cancer screening technique. BMC Cancer. 2004;4:76. doi:10.1186/1471-2407-4-76 [PMC free article: PMC533878] [PubMed: 15533242] [CrossRef]
- Shokar NK, Carlson CA, Weller SC. Informed decision making changes test preferences for colorectal cancer screening in a diverse population. Ann Fam Med. 2010;8(2):141-150. [PMC free article: PMC2834721] [PubMed: 20212301]
- Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening varies by race/ethnicity and screening strategy. Gastroenterology. 2010;138(5):S152.
- Myers RE, Hyslop T, Sifri R, et al. Tailored navigation in colorectal cancer screening. Med Care. 2008;46(9):S123-S131. [PubMed: 18725824]
- Myers RE, Sifri R, Daskalakis C, et al. Increasing colon cancer screening in primary care among African Americans. J Natl Cancer Inst. 2014;106(12):dju344. doi:10.1093/jnci/dju344 [PMC free article: PMC4817126] [PubMed: 25481829] [CrossRef]
- Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575-582. [PMC free article: PMC3360917] [PubMed: 22493463]
- Battaglia TA, Burhansstipanov L, Murrell SS, Dwyer AJ, Caron ASE. Assessing the impact of patient navigation: prevention and early detection metrics. Cancer. 2011;117(15 Suppl):3553-3564. [PMC free article: PMC4560256] [PubMed: 21780090]
- Lasser KE, Murillo J, Medlin E, et al. A multilevel intervention to promote colorectal cancer screening among community health center patients: results of a pilot study. BMC Fam Pract. 2009;10(1):37. [PMC free article: PMC2694166] [PubMed: 19480698]
- Cameron KA, Francis L, Wolf MS, Baker DW, Makoul G. Investigating Hispanic/Latino perceptions about colorectal cancer screening: a community-based approach to effective message design. Patient Educ Couns. 2007;68(2):145-152. [PubMed: 17517486]
- Jerant A, Kravitz RL, Fiscella K, et al. Effects of tailored knowledge enhancement on colorectal cancer screening preference across ethnic and language groups. Patient Educ Couns. 2013;90(1):103-110. [PMC free article: PMC3522756] [PubMed: 22985627]
- Mansfield C, Tangka FKL, Ekwueme DU, et al. Stated preference for cancer screening: a systematic review of the literature, 1990-2013. Prev Chronic Dis. 2016;13:E27. doi:10.5888/pcd13.150433 [PMC free article: PMC4768876] [PubMed: 26916898] [CrossRef]
- Canadian Breast Cancer Initiative. Investigation and Assessment of the “Navigator Role” in Meeting the Informational, Decisional and Educational Needs of Women With Breast Cancer in Canada. H39-663/2002E-PDF. Government of Canada. Published 2002. Accessed April 4, 2018. http://www
.publications .gc.ca/site/eng/297307/publication .html - Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract. 1995;3(1):19-30. [PubMed: 7704057]
- Till JE. Evaluation of support groups for women with breast cancer: importance of the navigator role. Health Qual Life Outcomes. 2003;1:16. https://www
.ncbi.nlm .nih.gov/pmc/articles/PMC155785/ [PMC free article: PMC155785] [PubMed: 12756054] - Oluwole SF, Ali AO, Adu A, et al. Impact of a cancer screening program on breast cancer stage at diagnosis in a medically underserved urban community. J Am Coll Surg. 2003;196(2):180-188. [PubMed: 12595043]
- HANYS Breast Cancer Demonstration Project. Breast Health Patient Navigator Resource Kit. Healthcare Association of New York State. Published September 2002. Accessed April 4, 2018. https://www.hanys.org/bcdp/resource_kits/upload/Complete-PNP-Kit-PDF.pdf [Link no longer works]
- Newman-Horm PA; C-Change. Cancer Patient Navigation: Published Information. C-Change; 2005.
- Guadagnolo BA, Dohan D, Raich P. Metrics for evaluating patient navigation during cancer diagnosis and treatment: crafting a policy-relevant research agenda for patient navigation in cancer care. Cancer. 2011;117(suppl 15):3565-3574. [PMC free article: PMC4818009] [PubMed: 21780091]
- Chen LA, Santos S, Jandorf L, et al. A program to enhance completion of screening colonoscopy among urban minorities. Clin Gastroenterol Hepatol. 2008;6(4):443-450. [PubMed: 18304882]
- Jandorf L, Gutierrez Y, Lopez J, Christie J, Itzkowitz SH. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J Urban Health. 2005;82(2):216-224. [PMC free article: PMC3456577] [PubMed: 15888638]
- Genoff MC, Zaballa A, Gany F, et al. Navigating language barriers: a systematic review of patient navigators' impact on cancer screening for limited English proficient patients. J Gen Intern Med. 2016;31(4):426-434. [PMC free article: PMC4803699] [PubMed: 26786875]
- Roland KB, Milliken EL, Rohan EA, et al. Use of community health workers and patient navigators to improve cancer outcomes among patients served by federally qualified health centers: a systematic literature review. Health Equity. 2017;1(1):61-76. [PMC free article: PMC5586005] [PubMed: 28905047]
- Sarfaty M, Wender R, Smith R. Promoting cancer screening within the patient centered medical home. CA Cancer J Clin. 2011;61(6):397-408. [PubMed: 22086728]
- Bazargan M, Ani C, Bazargan-Hejazi S, Baker RS, Bastani R. Colorectal cancer screening among underserved minority population: discrepancy between physicians' recommended, scheduled, and completed tests. Patient Educ Couns. 2009;76(2):240-247. [PubMed: 19150198]
- Lafata JE, Divine G, Moon C, Williams LK. Patient–physician colorectal cancer screening discussions and screening use. Am J Prev Med. 2006;31(3):202-209. [PMC free article: PMC4682196] [PubMed: 16905030]
- Weinstein ND, Sandman PM, Blalock SJ. The precaution adoption process model. In: Glanz K, Rimer BK, Viswanath, K, eds. Health Behavior and Health Education: Theory, Research, and Practice. Jossey-Bass; 2008:123-148.
- Powe BD. Fatalism among elderly African Americans. Effects on colorectal cancer screening. Cancer Nurs. 1995;18(5):385-392. [PubMed: 7585493]
- Franklin MD, Schlundt DG, Wallston KA. Development and validation of a religious health fatalism measure for the African-American faith community. J Health Psychol. 2008;13(3):323-335. [PubMed: 18420767]
- Moser A, Stuck AE, Silliman RA, Ganz PA, Clough-Gorr KM. The 8-item modified medical outcomes study social support survey: psychometric evaluation showed excellent performance. J Clin Epidemiol. 2012;65(10):1107-1116. [PMC free article: PMC4119888] [PubMed: 22818947]
- Vernon SW, Myers RE, Tilley BC. Development and validation of an instrument to measure factors related to colorectal cancer screening adherence. Cancer Epidemiol Biomarkers Prev. 1997;6(10):825-832. [PubMed: 9332766]
- Gustafson DH, Sainfort F, Eichler M, Adams L, Bisognano M, Steudel H. Developing and testing a model to predict outcomes of organizational change. Health Serv Res. 2003;38(2):751-776. [PMC free article: PMC1360903] [PubMed: 12785571]
- Stamatakis KA, Mcqueen A, Filler C, et al. Measurement properties of a novel survey to assess stages of organizational readiness for evidence-based interventions in community chronic disease prevention settings. Implement Sci. 2012;7(1):65. [PMC free article: PMC3418158] [PubMed: 22800294]
- Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. J Ment Health Policy Econ. 1996;425(2):7313.
- Ramsey S, Willke R, Briggs A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health. 2005;8(5):521-533. [PubMed: 16176491]
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis—principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17(1):5-14. [PubMed: 24438712]
Publications
- Myers RE, Stello B, Daskalakis C. Decision support and navigation to increase colorectal cancer screening among Hispanic primary care patients. Cancer Epidemiol Biomarkers Prev. 2019;28(2):384-391. [PubMed: 30333221]
Acknowledgment
Research reported in this report was [partially] funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (#AD-1306-01882) Further information available at:
Suggested citation:
Myers RE, Stello B, Sifri R, et al. (2019). Does Decision Support by Phone Increase Colorectal Cancer Screening in Hispanic Patients? Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/8.2019.AD.130601882
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Decision Support and Navigation to Increase Colorectal Cancer Screening Among Hispanic Patients.[Cancer Epidemiol Biomarkers Pr...]Decision Support and Navigation to Increase Colorectal Cancer Screening Among Hispanic Patients.Myers RE, Stello B, Daskalakis C, Sifri R, González ET, DiCarlo M, Johnson MB, Hegarty SE, Shaak K, Rivera A, et al. Cancer Epidemiol Biomarkers Prev. 2019 Feb; 28(2):384-391. Epub 2018 Oct 17.
- Increasing colon cancer screening in primary care among African Americans.[J Natl Cancer Inst. 2014]Increasing colon cancer screening in primary care among African Americans.Myers RE, Sifri R, Daskalakis C, DiCarlo M, Geethakumari PR, Cocroft J, Minnick C, Brisbon N, Vernon SW. J Natl Cancer Inst. 2014 Dec; 106(12). Epub 2014 Dec 6.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Review Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force[ 2016]Review Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task ForceLin JS, Piper MA, Perdue LA, Rutter C, Webber EM, O’Connor E, Smith N, Whitlock EP. 2016 Jun
- Review Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force[ 2021]Review Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task ForceLin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. 2021 May
- Does Decision Support by Phone Increase Colorectal Cancer Screening in Hispanic ...Does Decision Support by Phone Increase Colorectal Cancer Screening in Hispanic Patients?
Your browsing activity is empty.
Activity recording is turned off.
See more...
