This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
For patients with serious illness, communication about goals of care is associated with improved patient outcomes and reduced intensity of end-of-life care, but it is unclear whether interventions can improve this communication.
Objective:
To evaluate the efficacy of a patient-specific communication-priming intervention designed to increase goals-of-care conversations and targeting both patients with serious illness and their clinicians.
Methods:
Multicenter cluster-randomized trial of a patient-centered intervention implemented in outpatient clinics with physicians or nurse practitioners and their patients with serious illness. The intervention consisted of the Jumpstart-Tips form, a patient-specific communication feedback form that was generated from each patient's self-reported preferences for communication about his or her goals of care. The Jumpstart-Tips form was delivered to patients, family members, and clinicians before a routine visit between the patient and the clinician. The comparison group received usual care. The study team assessed the effectiveness of the intervention by following patients until death or up to 6 months. The primary outcome was patient-reported occurrence of a goals-of-care conversation during a target outpatient visit. Secondary outcomes included clinician documentation of a goals-of-care conversation in the medical record and patient-reported quality of communication (mean rating of 4 items) at 2 weeks, as well as patient assessments of goal-concordant care at 3 months and patient-reported symptoms of depression (Patient Health Questionnaire-8) and anxiety (General Anxiety Disorder-7) at 3 and 6 months. Analyses were clustered by clinician and adjusted for baseline patient characteristics.
Results:
We enrolled 132 of 485 potentially eligible clinicians (27% participation) and 537 of 917 eligible patients (59% participation). Clinicians were randomized to the preconversation communication-priming intervention (n = 65) or usual care (n = 67). Of the patients, 249 received the intervention and 288 received usual care. The intervention was associated with a significant increase in a goals-of-care discussion at the target visit (74% vs 31%; p < .001) and increased medical record documentation of a goals-of-care discussion (62% vs 17%; P < .001), as well as increased patient-rated quality of communication (4.6 vs 2.1; P = .010). Patient-assessed goal-concordant care did not increase significantly overall (70% vs 57%; P = .077) but did increase for the prespecified subgroup of patients with stable goals between the 3-month follow-up and the last prior assessment (74% vs 57%; P = .029). Patients' symptoms of depression or anxiety did not differ between groups at 3 or 6 months (P > .1 for all).
Conclusions:
This intervention increased the occurrence, documentation, and quality of goals-of-care communication during routine outpatient clinic visits and was associated with increased patient-assessed goal-concordant care at 3 months for patients with stable goals between the 3-month follow-up and the last prior assessment. The intervention was not associated with any change in symptoms of anxiety or depression. Limitations include a relatively low participation rate among clinicians and the fact that the study took place in only 1 region of the United States with a high proportion of white, non-Hispanic patients. Although additional studies are needed to evaluate whether this communication is associated with changes in health care delivery, the use of a simple communication tool prepared from patient responses to a discrete set of questions and provided to clinicians and patients before a routine clinic visit holds promise for health care systems seeking to increase goals-of-care communication for patients with serious illness.
Background
Overview
Four decades of research on palliative and end-of-life care in the United States indicate that people who are dying often spend their final days with a significant burden of pain and other symptoms and receive care they would not choose. One of the most important components of communication about palliative care is the exploration, identification, and enactment of each patient's goals of care. Patient–clinician communication about goals of care is an important focus for improving care for 3 reasons: (1) When it occurs, it is associated with improved quality of life and reduction in intensive life-sustaining therapies at the end of life; (2) physicians frequently do not have these goals-of-care discussions; and (3) our preliminary studies suggest that a relatively simple patient-specific intervention can increase the occurrence and quality of patient–clinician communication about goals of care. In this randomized trial, we used an innovative, patient-centered intervention to improve communication among patients with serious illness, their families, and their clinicians. The intervention identified patient-specific preferences for communication about goals of care and fed this information back to the patient, family, primary clinician, and interprofessional (IP) clinical team. We implemented this intervention in clinical practice in a way that may be feasible and generalizable for broad implementation as a means for structuring and supporting communication among patients, families, and clinicians.
Background
Good communication among clinicians, patients, and patients' families is critical to improving palliative care.1-3 In focus groups of patients and families, we found that quality of communication is a key domain of clinician skill in palliative care.4 Despite its importance, the quality of clinician communication about palliative care is suboptimal in many areas, including discussions about prognosis,5-8 advance care planning and goals of care,9-13 and shared decision making.14 Some recent studies9,15,16 have suggested promise for communication interventions, showing improvement in the quality of life and care while also reducing high-intensity care at the end of life. For this promise to be realized, we need to develop feasible ways to translate effective communication about palliative care into routine clinical practice.
Although it is important to expand the field of specialty palliative care, it is also critical that a broad spectrum of clinicians—including primary care providers and other specialty clinicians—are able to provide high-quality primary palliative care.17 This need is driven by many circumstances. First, the number of patients with chronic, life-limiting illness is increasing as the US population ages, and there are not enough palliative care specialists to provide care for all these patients; it is neither necessary nor even desirable for all of these patients to be seen by a palliative care specialist.18 Second, nonpalliative care clinicians play a central role in palliative care even for patients who see palliative care specialists. For example, clinicians who have not had palliative care specialty training diagnose terminal illness, discuss prognosis, and initiate transitions from curative-focused to palliative-focused care.19 Thus, ensuring that high-quality palliative care is provided by a variety of clinicians is important to improve the quality of end-of-life care even in the setting of a rapidly growing palliative care specialty.18 Discussions about the individual patient's goals of care are a key part of this communication.20-23
Most patients with chronic, life-limiting illness want to talk to their clinicians about goals of care, but only a small proportion do so.15,24,25 This communication, when it occurs, is associated with increased quality of life for patients and decreased psychological symptoms for patients and family members.9,15 Approaches to increasing this communication have included interventions that focus on training clinicians to conduct goals-of-care communication (clinician-facing educational interventions)1-5,26-30 and decision aids or tools that support patients and family members in considering their goals of care and conducting advance care planning (patient-facing interventions).6-10,31-35 Research has suggested that structured communication tools may increase the frequency of discussions about and completion of advance directives and improve concordance between the care desired and the care received by patients; however, both the quality and results of previous studies have varied, leaving the effectiveness of the communication tools unclear.11-13,36-38 Our intervention differs from clinician training and patient-facing decision aids by obtaining specific information from patients to prompt and guide both patients and clinicians in a discussion about goals of care.
Our research into communication about palliative care has been guided by the 4 key questions by which PCORI defines patient-centered outcomes research. Patients and their families want to know (1) what to expect as chronic, life-limiting illness progresses; (2) their options and the potential benefits and harms; (3) what they can do to improve the outcomes that are most important to them; and (4) how clinicians and care delivery systems can help them make the best health care decisions.
To address these questions, it is important to increase patient–clinician communication about prognosis and goals of care and to incorporate family members into this discussion whenever possible, as they often act as surrogate decision makers for the patients.39-42 This communication must include information about benefits and harms of different treatment approaches and must also facilitate shared decision-making. Patients' values, goals, and preferences about end-of-life care vary dramatically; improved communication about these matters among patients, family members, and clinicians can give patients the opportunity to think about them and to have their preferences inform the care they receive. Effective communication about goals of care should occur early enough in a patient's illness so that he or she can participate; patients say they want to have these discussions when they are feeling well enough to have meaningful conversations.4,43 Many patients also say they want to have this communication with their current physicians.4,43-45
We designed our intervention to change the ways in which in patient-centered information is provided by health care systems to clinicians, with the goal of facilitating and enhancing communication about goals of care among patients with serious illness, their family members, and the clinicians who are caring for them. We focused on patients with chronic, life-limiting illness. Our primary research question was the following: Does this patient-centered communication intervention (which we called Jumpstart-Tips), improve outcomes for patients with chronic, life-limiting illness and their families?
Aim 1: Evaluate the effect of the intervention on facilitating and improving patient–clinician communication about the goals of care for patients with chronic, life-limiting illness. The primary outcome was the occurrence of patient–clinician communication about goals of care. We also assessed the intervention's effect on patients' ratings of the quality of this communication.
Hypothesis 1: The intervention will increase the occurrence of communication about goals of care and will improve the quality of this communication.
Aim 2: Assess the effect of the intervention on ensuring that patients with life-limiting illness receive the care they want as measured by concordance between the care patients want and the care they receive; use of palliative care services for patients with unmet palliative care needs; and reduced use of intensive life-sustaining treatments at the end of life for patients who do not want such treatments.
Hypothesis 2: The intervention will improve patient-centered care as measured by increased concordance between patients' wishes for care and care received; increased palliative care referrals for those who want such referrals; and reduced use of unwanted life-sustaining treatments at the end of life.
Aim 3: Evaluate the effect of the intervention on patients' symptoms of anxiety and depression in the context of receiving care for a life-limiting illness.
Hypothesis 3: The intervention will reduce the burden of psychological symptoms for patients.
Participation of Patients and Stakeholders
Our stakeholders included patients with serious illness; family members of patients; clinicians; and community members with an interest in education, research, or promoting palliative care. Our primary interaction with these stakeholders was through the Community Advisory Board (CAB) of the Cambia Palliative Care Center of Excellence (PCCE) at the University of Washington. CAB membership has averaged 20 to 25 people, with a core group of 10 to 12 who attended nearly all the meetings during the period of this study. All stakeholder groups were represented on the board (see Appendix A for additional details).
The CAB had a notable impact on study operations by reviewing and discussing study materials. This included feedback on definitions, wording, and formatting of study recruitment letters to potential participants, questionnaires for patients and family members, and the Jumpstart-Tips forms that were part of the intervention. The feedback included a suggestion that less research jargon be used and enumerated some possible concerns. The CAB was also instrumental in refining the introductory Jumpstart-Tips videos that explained the intervention to patients, family members, and clinicians.
Some of the CAB's contributions to study quality came in the form of suggestions and feedback to the researchers about the acceptability/understandability of survey items, and changes and improvements to patient recruitment strategies. Stakeholders discussed how patients and families might interpret survey items, suggested changes in language or wording to ensure appropriateness, proposed strategies for reaching family members to increase that group's study participation, and supported and validated approaches that the research staff were already using to give them confidence when contacting prospective patient and family study participants. The researchers shared the study results with the CAB members, discussing each aim and the findings associated with that aim. The CAB had important questions about our findings and conclusions, including the likely effect of patients who were approached but did not participate and of nonresponses from participants.
The researchers benefited from hearing and integrating stakeholder perspectives into research questions, materials, procedures, and conclusions. These perspectives were not only about usability and acceptability but also about substantive concerns such as the importance of spirituality and religiosity for patients facing the end of life, including their caution that we should not assume that spirituality and religion are important to all patients. We also appreciated the CAB's feedback about the potential for bias and ways to maximize the generalizability of our findings.
Recruitment and retention of the CAB members was an important focus of our engagement activities. CAB members were frequently asked for their input on community engagement; that is, recruiting more people with serious illness and their family members to the CAB itself. The Cambia PCCE provided information about the CAB at various public events, and volunteers distributed fliers about CAB membership to local organizations, libraries, and centers. CAB members regularly brought family members and colleagues along with them to meetings. Strategies that worked well to retain stakeholders included having regular meetings, so stakeholders could plan in advance, providing parking or covering transportation costs to facilitate attendance, sending materials ahead of the scheduled meeting to ensure that board members had time to review them, and maintaining a website on which the researchers and stakeholders could share information.
One of the challenges we faced in this study was identifying patients with serious illness (our principal recruitment population) who felt well enough to participate as stakeholders on the CAB. Although we reached out to other organizations (eg, American Heart Association, American Lung Association), made calls and presentations, and posted announcements at several community locations, these efforts did not yield many additional CAB members who were patients with serious illness. Thus, most of the CAB members were relatives of people who were experiencing or had died from serious illness.
Methods
Study Overview
This study evaluated a patient-centered intervention (Jumpstart-Tips) to improve goals-of-care communication among patients with chronic, life-limiting illness; their families; and their clinicians. The intervention identified patient-specific preferences for communication about goals of care, as well as barriers to and facilitators of this communication. This information was shared with the patient, family, primary clinician, and IP team in a short feedback form. The specific aims for this research were to assess the intervention's effect on the following: (1) the occurrence and quality of communication; (2) concordance between care received and desired care; (3) referrals to palliative care service for patients who preferred care focused on comfort and reduced provision of unwanted life-sustaining therapies; and (3) patients' and family members' symptoms of anxiety and depression in the context of care for a life-limiting illness.
Study Design
The study was a cluster-randomized trial of a patient-centered intervention implemented at the level of the health care system. The intervention consisted of the Jumpstart-Tips form, a patient-specific communication feedback form based on each patient's preferences for communication about goals of care. The form was provided to patients, family members, primary clinicians, and IP health care team members. (The intervention is described in detail later in this document.) Primary clinicians included primary care and subspecialty physicians, as well as nurse practitioners who cared for these patients in a primary clinician role, in which communication about goals of care would be appropriate. The design of the intervention also included the IP team involved in the patients' care. The unit of randomization was the primary clinician. Patients, family members, and IP team members were clustered under the primary clinician. To evaluate the impact of the intervention on care as patients became increasingly ill and to assess its durability, we followed patients for 6 months or until their death if that occurred within 6 months of study enrollment. We evaluated the intervention through its association with the occurrence and quality of goals-of-care communication for all patients and specifically for patients who did not indicate at baseline that they wished to avoid a goals-of-care discussion.
Additional patient- and family-centered outcomes included concordance between the care patients said they would like and the care they actually received, the use of palliative care if desired, and the receipt of high-intensity life-sustaining treatments. Finally, we measured whether the intervention affected psychological symptoms in patients. The purpose of assessing symptoms of anxiety and depression was not to attempt to diagnose these clinical syndromes but rather to evaluate the psychological distress that can be caused by poor-quality communication with clinicians. An established literature shows that interventions to improve communication can reduce symptoms of anxiety and depression.9,46,47 In this study, psychological symptoms were conceptualized as a marker of distress due to poor communication. We originally planned to examine psychological symptoms among family members too, but low participation in this group prohibited robust analysis of family symptoms.
The intervention was based on self-efficacy theory,48-50 which has guided many interventions designed to modify patient51,52 and clinician behaviors.53-55 In this theory, the impetus for change resides in a person's efficacy expectations or “confidence in one's ability to take and persist in action.”56 These expectations reflect people's beliefs about how capable they are in performing a task. We designed the Jumpstart-Tips intervention to promote the expectations of clinicians and patients for having discussions with each other about goals of care. External supports, such as institutional expectations and automatic reminders for goals-of-care discussions, are a part of self-efficacy theory and were components of this intervention, contributing to self-efficacy for clinicians and patients.
In a previous study, we piloted the basic features of the intervention evaluated here, demonstrating that the intervention can be implemented in an outpatient setting and is associated with increased occurrence and quality of patient–physician communication about goals of care.25 The study was a cluster-randomized trial of patients with chronic obstructive pulmonary disease (COPD), randomizing clinicians from primary care and chest clinics at the Veterans Affairs Puget Sound Healthcare System (an entity separate from University of Washington Medicine).
We tested methods for the current study during a pilot phase of an abbreviated intervention that included screening, recruitment, enrollment, intervention and target visit, and completion of post-visit surveys. Six clinicians, 4 patients, and 1 family member completed activities in the pilot, and we made the following changes in response to challenges encountered: (1) revising survey instructions that were hard to understand, (2) revising survey response options for additional clarity, and (3) revising the format of Jumpstart-Tips forms for clinicians.
Participants
The main study participants were primary clinicians and patients. Patients identified family members and primary clinicians identified team members/IP clinicians for possible participation. Inclusion criteria are detailed in the following sections. Reasons for exclusion for all participant groups included inability to speak English, legal or risk management concerns, and physical or cognitive limitations that prevented a person from completing research activities and questionnaires. We excluded non–English speaking people because most of our study questionnaires have not been translated and validated in other languages. For patients and family members, participation in the pilot phase was also an exclusion criterion in the full study. Participants were recruited from outpatient clinics within the University of Washington Medicine health care system and from Swedish Medical Center Primary Care clinics (see the “Study Setting” section).
Primary clinicians (n = 132 enrollees, 124 of whom had patients who enrolled and completed their target visit): Eligible primary clinicians included all clinicians who provided ongoing primary or specialty care to potentially eligible patient populations. This included physicians (eg, family medicine, internal medicine, oncology, pulmonology, cardiology, gastroenterology, nephrology, geriatrics), nurse practitioners, and physician assistants. A primary clinician was any clinician who would be expected to discuss goals of care with eligible patients. Consecutive clinicians at UW Medicine were identified via publicly available lists of providers in each practice or by screening the clinic's electronic health record to identify clinicians with potentially eligible patients in their clinic panels. Clinicians at Swedish Medical Center were introduced to the study at staff meetings and volunteered at that time to be contacted for participation. EHR screening required a waiver of consent and waiver of HIPAA authorization. Potential primary clinicians were identified as those having at least 5 eligible patients. When it was not possible for study staff to screen clinicians for eligibility (eg, no data were available in the EHR repository), we directly contacted potential clinician participants and allowed them to review our minimal criterion (ie, sufficient eligible patients to allow enrollment of 6 patients over a 12-month period) and indicate whether they met this criterion. Clinicians were asked to confirm their eligibility at recruitment. Clinicians were initially approached by mail or email, with follow-up by telephone or in person.
Clinicians met in person with study staff to complete enrollment. At these enrollment meetings, clinicians reviewed study materials and completed written informed consent and, when possible, baseline questionnaires. Questionnaires were self-administered and were completed on paper or online, depending on the clinician's preference. The baseline questionnaire included the following:
- Competence in Communication about End-of-Life Care: 17-item self-assessment of perceived competency, developed and validated previously57
- Demographics, including year of birth, discipline/specialty, ethnicity, and race
Patients (n = 537 enrollees, of whom 494 completed their target visit): We enrolled up to 6 patients per primary clinician. Consecutive eligible patients, identified from the EHR or clinic records, were required to be under the care of a participating primary clinician, to be aged 18 years or older, to have had 2 or more visits with the primary clinician in the past 18 months, to have an upcoming appointment, and to meet at least 1 of the diagnostic criteria. The diagnostic criteria included the following, with the goal of identifying patients with a median survival of about 2 years:
- Metastatic cancer or inoperable lung cancer
- Chronic obstructive pulmonary disease with the forced expiratory volume in 1 second (FEV1) values <35% predicted or oxygen dependence
- Restrictive lung disease with a TLC <50% predicted
- New York Heart Association class III or class IV heart failure
- Left ventricular assist device or implantable cardioverter defibrillator with age of 65 years or older
- Child's class C cirrhosis or Model for End-Stage Liver Disease score > 17
- Dialysis-dependent renal failure and either diabetes or a serum albumin <2.5
- Pulmonary arterial hypertension with 6-minute walking distance <250
- Restrictive lung disease (idiopathic pulmonary fibrosis, interstitial lung disease) with TLC <50%
- Cystic fibrosis with FEV1<30%
- Aged 75 years or older with diagnosis of at least 1 of the life-limiting, chronic illnesses noted above, although possibly of lesser severity
- Hospitalization from any cause within the past 18 months with diagnosis of at least 1 of the life-limiting, chronic illnesses noted above, although possibly of lesser severity
- Aged 90 years or older
- Charlson index score ≥ 6
Exclusion criteria included factors that would make the patient a poor fit for this study, such as legal or risk management concerns or physical or mental limitations that would prevent the person from completing the research activities. Using the EHR and the eligibility criteria, study staff identified the panel of eligible patients being cared for by each participating clinician. They collected the following information from the EHR: diagnoses, dates of recent and upcoming clinic appointments, demographics (gender, ethnicity, race), birth date, medical record number, name, and contact information.
Study staff contacted eligible patients by mail and by telephone. Interested patients met the study staff at enrollment meetings, where they reviewed study materials and completed written informed consent and HIPAA authorization. Patients whose clinicians had been assigned to the intervention group viewed the video that explained the Jumpstart-Tips form and how it might be used. Baseline data were collected at the time of enrollment via self-administered questionnaire or study-staff-administered interview, according to the patient's preference. The baseline patient questionnaire included the following:
- Preferred vs Actual Care Intensity: 2 items from the SUPPORT study addressing preferred and actual focus of care (life extension vs comfort) for use in evaluating goal-concordant care and 2 items about preferences regarding cardiopulmonary resuscitation (CPR)
- Communication Barriers and Facilitators: 16 items assessing patient-specific barriers to and facilitators of goals-of-care communication
- Quality of Communication (QOC_eol): 7 items assessing the quality of the clinician's communication about issues relevant to the patient's end-of-life period
- Trust: 5 items assessing the respondent's trust in the clinician
- Patient Health Questionnaire (PHQ-8): assessing symptoms of depression
- Generalized Anxiety Disorder (GAD-7): assessing symptoms of anxiety
- Religion and Spirituality: 4 items assessing respondent's religiosity
- Demographics, including year of birth, gender, marital status, education, self-perceived general health status, ethnicity, race, and income
Families (n = 119): At each patient's enrollment visit, we asked the patient to identify a family member who was involved in his or her medical care if such a person existed. For the purpose of this study, family member was not confined to legal next of kin or even to immediate family. If an eligible family member was available at the time of the patient's enrollment, he or she was approached by study staff about participating in the study; otherwise, the family member was approached by mail or telephone. If he or she was recruited and enrolled in person, written consent was obtained, and baseline data collected during the enrollment visit. We mailed consent forms and questionnaires to those recruited by mail or phone. If a family member returned a questionnaire but no signed consent form, we obtained a Waiver of Written Documentation of Consent. Questionnaires were usually self-administered. The baseline questionnaire included the following:
- Preference for Intensity of Care at End of Life: items addressing the patient's care preferences
- Trust: 5 items assessing the family member's trust in the patient's enrolled clinician;
- PHQ-8: assessing the family member's symptoms of depression
- GAD-7: assessing the family member's symptoms of anxiety
- Demographics, including year of birth, gender, marital status, education, self-perceived general health status, relationship to patient, cohabitation with patient, ethnicity, race, religiosity, and income
IP team members (n = 4): Eligible IP team members included nurses, social workers, and other clinicians who were part of an enrolled primary clinician's clinic and provided care to eligible patients. We attempted to enroll all team members identified by the primary clinician, but most clinicians did not have an IP team member working with eligible patients. IP team members were approached by mail, email, or in person. They received an introductory mailing that contained copies of the consent form and survey materials, along with a return envelope. If they returned the questionnaire but neglected to include a signed consent form, a Waiver of Written Documentation of Consent allowed us to use their data. Baseline data for team members were collected at the time of enrollment. Questionnaires were self-administered. The baseline questionnaire included the following:
- Skills and Behavior in Practice: 14-item self-assessment of demonstrated communication skills
- Demographics, including year of birth, discipline/specialty, ethnicity, and race
Follow-up efforts were made with all potential participants who did not respond to initial contact efforts. These efforts included second mailings of materials and additional telephone calls (all participant types) or emails (clinicians only). When potential enrollees declined to participate, study staff attempted to ascertain the reason for refusal, although a reply of “not interested” was not questioned further. Stated reasons for nonparticipation included too burdensome (eg, does not want to discuss end-of-life issues); too sick; privacy concerns; too busy/no time; topic does not apply to me; no need for something like this.
When the study was initiated, we based sample size calculations for the primary outcome on data from our previous trial.25 We estimated power to detect a significant difference in the occurrence of a goals-of-care discussion among patients who did not report that they wanted to avoid such a discussion. Based on the previous study, we estimated that two-thirds of patients would want a goals-of-care discussion with their clinician and that 12.8% of control patients and 30.0% of intervention patients would report on their postvisit follow-up that discussions had occurred at the target visit. Power calculations, based on multilevel modeling principles specifying randomization at level 2 and outcomes at level 1, indicated 95% power for detecting the targeted difference with a sample of 120 clinicians, each having 6 enrolled patients, with 4 desiring end-of-life communication (ie, a total of 120 clinicians and 720 patients, with 480 patients desiring a discussion). Because of difficulties with patient enrollment, we performed an interim examination of the overall proportion of patients reporting a goals-of-care discussion. After finding higher proportions than we estimated, we modified our target sample size to 120 clinicians and 500 patients. This change was approved by the data and safety monitoring board and PCORI. All power calculations confirmed that the targeted sample sizes would provide adequate power for assessing secondary outcomes.
In this clinician-level cluster-randomized trial, we randomized clinicians at a 1:1 ratio. Randomization was stratified by site, with randomly assigned variable block sizes, using computer-generated random number sequences. We were unable to blind clinicians or patients to the intervention, but study staff evaluating outcomes were blinded to random assignment groups.
Intervention and Control Groups
The Jumpstart-Tips were adapted from a similar intervention evaluated in an efficacy trial among patients with COPD.25 The intervention we used was modified in response to feedback from patients and clinicians in the previous trial, including simplifying the form, reducing the amount of information included from patient surveys, and updating the tips used to guide goals-of-care discussions. In this trial, we chose to compare the Jumpstart-Tips intervention against usual care (as opposed to a comparative effectiveness trial) for the following reasons: (1) At the time this study started, no other outpatient communication interventions for goals-of-care discussions had been documented to improve patient-centered outcomes, and (2) as there were few other programs in place to increase communication, usual care was the most appropriate comparator from the perspectives of the health care system implementing the intervention and the individual patients and family members who participated. At the time of the patient's enrollment, the target clinic visit was identified as the next scheduled visit with the enrolled clinician. The intervention took place during this visit and, for both intervention and control patients, was the trigger point for scheduling the collection of follow-up data. Tracking target visits consisted of daily monitoring for previsit mailings (to intervention patients) or telephone calls (to control patients to provide comparable contact and encourage comparable patient retention in the 2 arms). Before the previsit contact, the study staff confirmed the status of the target visit via the patient's EHR.
Intervention
The intervention, based on self-efficacy theory, consisted of a short communication feedback form (Jumpstart-Tips) and a video introduction to the use of the form. The form—provided to the clinician, patient, and family member before the target visit—was a 1-page document specifying patient-specific preferences for communication about goals of care, communication barriers and facilitators, and patient preferences for CPR, all as reported by the patient on the baseline questionnaire. The form was tailored to each recipient (patient, clinician, team member, family member) to support the communication tasks the recipient would be positioned to address. For example, the form for the clinician included cues to initiate discussion and suggestions for addressing reluctance with patients who did not want to talk about goals of care; patient and family forms included tips on how to bring up topics of concern with clinicians and with each other. (See Appendix B for examples of Jumpstart-Tips forms.)
Upon receipt of the patient's baseline questionnaire, the study staff created a patient-specific Jumpstart-Tips form, using a matrix of standard responses conditioned on the patient's own responses (see Appendix C). The individual Jumpstart-Tips form included whether the patient had talked to the clinician about goals of care and whether future discussions of this type were desired, the patient's current goals of care, and the primary barrier to or facilitator of such discussions. Clinicians who were randomized to the intervention were asked to view the instructional video. If the video was not shown during the enrollment visit, links to the video and instructions for viewing were included in an email announcing their randomization assignment.
Jumpstart-Tips forms for the clinicians were provided via secure email or fax approximately 2 days before the patient's target clinic visit and again the morning of the visit. In a few cases, forms were delivered in person to the clinic. A hyperlink to the training video was included with Jumpstart-Tips forms and reminder emails, to encourage review. Clinicians in the intervention group had the option of postponing discussion about end-of-life care to a subsequent visit if the timing was not appropriate at the target visit.
In addition to information about patient-specific barriers to and facilitators of goals-of-care discussion, the Jumpstart-Tips form alerted the clinician to patients who might benefit from referral to a palliative care specialist. A combination of 3 responses on a patient's baseline questionnaire triggered such an alert: (1) a desire to talk about end-of-life care; (2) no previous discussion of end-of-life care with the enrolled clinician; and (3) an orientation toward ensuring comfort rather than extending life.
Enrolled IP team members received Jumpstart-Tips forms identical to those received by the clinician. The goals of incorporating the IP team were to use the team to reinforce discussions between the clinician and the patient and to provide support for patients and their families.
Jumpstart-Tips forms were sent by mail to patients and participating family members before the target visit and were provided in person at clinic visits when feasible. The pre-visit mailing included the following: a cover letter, a link to view the Jumpstart-Tips video online or a summary (transcript) of the video, and a study steps checklist (ie, a list of time points for contact—baseline, target visit, follow-up). The checklist was included so participants would know what to expect next and how long their participation would continue.
Control
The control arm consisted of usual care plus survey completion. All participants randomized to the control group completed the same surveys at all data collection points and received phone calls to remind them about upcoming target visits and survey mailings. Patient-specific information in a Jumpstart-Tips form was not provided to patients, family members, or clinicians. After the target clinic visit, the follow-up survey schedule began (2 weeks, 3 months, 6 months). Timing and materials were the same for participants in the intervention and control arms.
Study Outcomes
All study outcomes have been validated among patients with serious illness. Appendix D includes a timeline showing the time points for the measurement of each outcome, and Appendix E includes the outcome instruments.
Primary Outcome Variable
Occurrence of communication about goals of care at the target visit (Aim 1)
We evaluated this outcome with patient reports from the 2-week follow-up questionnaire, using a previously validated dichotomous item.24,25,58 In addition to patient reports that a goals-of-care conversation took place, we evaluated whether the outcome occurred according to EHR documentation of whether any of the following topics were discussed at the target visit: advance care planning, end-of-life treatment preferences, physician orders for life-sustaining treatment (POLST) forms, prognosis, palliative care, or hospice. We examined the results of the study for both versions of the outcome with the full sample and with patients who had not registered an objection on their baseline questionnaire to future goals-of-care discussions.
Secondary Outcome Variables
- Quality of communication about end-of-life care (Aim 1): We assessed this with 7 items pertaining to end-of-life communication, drawn from the Quality of Communication (QOC) survey. We developed the QOC from qualitative interviews and focus groups with a diverse set of patients, family members, and clinicians.24,43,58 The QOC_eol subscale was based on patients' ratings of 7 aspects of the clinician's communication with the patient, with ratings potentially ranging from 0 (worst) to 10 (best) and with an additional response option indicating that the clinician had not addressed a particular aspect of communication.58 We selected 4 of the items as most related to this intervention, recoded the 0 to 10 ratings to values of 1 to 11, and imputed a value of 0 if the patient indicated that the clinician did not cover the item. We used confirmatory factor analysis (CFA) to evaluate the 4 items for unidimensionality and scalar measurement invariance between groups (intervention and control) and over 2 assessments (baseline and 2 weeks). The items were defined as censored from below (because of high frequencies of “did not do”) and were analyzed with Tobit regression models, constraining each indicator's loading and intercept to equality over the 2 groups and 2-time periods. This 4-indicator latent variable model showed excellent fit with the observed data: Based on the χ2 test of fit, with P > .05 required as evidence of nonsignificant misfit, the model produced P = .7773 when based on all cases and P = .8031 when based on patients with complete data. In addition to the 4-indicator latent construct, we tested each of the 7 end-of-life-communication items (recoded to the 0-11 scale) as separate outcomes, defined as censored from below and analyzed with Tobit regression.
- Goal-concordant care (Aim 2): We assessed concordance using patients' responses to 2 questions on the 3-month follow-up questionnaire. The questions, taken from the SUPPORT study,59 asked (1) whether the patient preferred care focused on extending life or care focused on ensuring comfort, and (2) whether the patient perceived his or her current care as focused on extending life or ensuring comfort. Each of the questions also included an “I'm not sure” response. Patients were placed in the goal-concordant group if the current focus of their care matched their preference; they were in the goal-discordant group if the current focus of care did not match their preference or they responded “I'm not sure” to either question.
- Referral to palliative care services (Aim 2): We assessed referral to palliative care services for the entire 6-month follow-up period, using the EHR. We collected data separately from outpatient visits and inpatient stays, with a summary measure combining referrals during both types of visits. We evaluated these binary outcomes for all patients and for patients who potentially had unmet palliative care communication needs. We identified those with unmet needs by positive responses to 2 questions on either the 3-month questionnaire or the last questionnaire completed before that; the questions involved whether they preferred care focused on comfort and whether they would like to have future goals-of-care discussions with their participating clinician.
- Avoidance of life-sustaining therapies (Aim 2): Review of the patient's EHR determined whether there was any use of 3 specific life-sustaining therapies during the 6-month follow-up period: (1) admission to an ICU; (2) receipt of CPR; or (3) mechanical ventilation. The review produced a single binary variable for each patient (0 = some use of 1 or more of the 3 therapies; 1 = no use of any of the therapies). Analysis of this outcome included a consideration of patients' preferences for care.
- GAD-7 (Aim 3): We evaluated patient anxiety symptoms using the GAD-7, a self-report 7-item measure of anxiety symptoms. In a criterion-standard study, the GAD-7 demonstrated excellent psychometric characteristics.60,61 The full set of 7 GAD items was not unidimensional in our study sample, so we investigated whether a construct based on a smaller number of indicators might provide an appropriate latent measure. Using exploratory factor analysis in a confirmatory factor analysis framework (E/CFA), beginning with all 7 items and sequentially removing items that contributed maximally to misfit, we identified a 2-indicator construct (items 1 and 3, defined as ordered categorical variables and analyzed with probit regression) that—with measurement invariance imposed between groups (intervention and control) and over 3 time periods (baseline, 3 months, 6 months)—produced an χ2 test of fit with P = .7687 for all cases and P = .6227 for cases with complete data.
- Patient Health Questionnaire (Aim 3): We assessed symptoms of depression among patients using the PHQ-8, an 8-item questionnaire that is appropriate for primary care and general populations.62 As with the GAD-7, the full set of items from the PHQ-8 did not measure a unidimensional construct in this sample, so we used CFA to evaluate the PHQ-2, a 2-item measure of depressive symptoms.63,64 A latent construct based on these 2 items—with measurement invariance imposed between groups and over time—showed nonsignificant misfit to the data (P for the χ2 test of fit = .0897 for all cases and P = .1609 for cases with complete data). These findings support the use of the latent construct as a measure of depressive symptoms.
Qualitative Outcome
In response to suggestions from PCORI staff, we added semistructured interviews to this study with the goals of exploring recruitment challenges for family members and IP clinicians, obtaining feedback about the intervention, and identifying participant suggestions for improvements to the intervention. The interviews were tailored to each type of respondent (patients, families, clinicians) and included 5 to 8 questions, depending on the respondents' experiences.
Study Setting
Subjects were recruited and enrolled from outpatient clinics in the UW Medicine health care system and the Swedish Primary Care clinics.
- Harborview Medical Center (HMC) is owned by King County and operated by the University of Washington. Its patient population is diverse and includes racial/ethnic minorities and individuals from a wide range of socioeconomic and cultural backgrounds. HMC's outpatient clinics include more than 200 primary and specialty physicians, nurse practitioners, and physician assistants who care for more than 25 000 patients per year. Clinics include nephrology, pulmonary, cardiology, geriatrics, hepatology, and oncology, as well as primary care.
- The University of Washington Medical Center is an academic medical center providing tertiary and quaternary care for the region and nationwide and is a leading provider of primary and specialty care in Seattle and King County. It provides an integrated system of outpatient clinics staffed by more than 300 primary and specialty care physicians, nurse practitioners, and physician assistants who serve more than 30 000 patients a year.
- ?Northwest Hospital and Medical Center (NHMC) is a full-service, nonprofit community hospital located in north Seattle. NHMC and its affiliates provide an integrated system of outpatient clinics staffed by more than 600 primary and specialty care physicians, nurse practitioners, and physician assistants, with more than 500 000 patient visits each year.
- Valley Medical Center is an acute care hospital and clinic network serving more than 600 000 residents, making it the largest nonprofit health care provider in southeast King County. The Valley Medical Provider Group and its affiliates consist of 600 physicians and other health professionals staffing a network of primary care clinics and more than a dozen specialty clinics, with more than 330 000 patient visits yearly.
- Swedish Medical Center (SMC) is the largest nonprofit community health care system in the greater Seattle area that is not part of the UW Medicine system. It includes a network of more than 100 primary care and specialty clinics throughout the greater Puget Sound area. Swedish Primary Care has 200-plus primary care physicians at more than 30 clinics.
Time Frame for the Study
Self-report Questionnaires
In addition to baseline questionnaires, patients and family members in both arms of the study completed questionnaires at 3 points after the target visit: 2 weeks, 3 months, and 6 months. Clinicians completed a post–target visit survey for each patient. We chose this time frame to enable us to assess the early effects of the intervention on patients who might become increasingly ill over time and to test for durability of the effects, all without being overly burdensome. For the primary outcome, we selected a specific time point: 2 weeks.
EHR Review
We assessed health care utilization at the end of the 6-month follow-up period, allowing us to assess the impact of the intervention on utilization as well as its effect on outcomes reported on participant questionnaires. Abstraction included the following elements: disease characteristics, including type and severity; occurrence and documentation of advance care planning and other communication events relevant to the end of life; occurrence and timing of hospitalizations, including acute care and ICU stays; receipt of life-support measures, including CPR and mechanical ventilation; and occurrence of specialty referrals, consults, and visits, including palliative care, social work, spiritual care, and ethics.
Qualitative Data
Participants in the intervention group were approached for qualitative interviews upon completion of all study activities. We did not want this interview to affect the intervention outcomes, so we did not approach subjects until all questionnaires had been completed. The qualitative interviews occurred between 6 and 19 months after the interviewees' target visits.
Data Collection and Sources
Clinician Surveys
To encourage clinicians in both study arms to complete the short post–target visit survey for each participating patient, we offered a variety of methods (paper, online, phone) and sent reminders by telephone, fax, mail, and email. We encouraged continuing clinician involvement by providing regular study-status updates and a $5 coffee card for each survey.
Patient and Family Member Surveys
Study staff endeavored to keep enrolled patients and family members engaged through frequent contact throughout the study period. After enrollment, subjects received letters or telephone calls before their target visits and before follow-up surveys were mailed. Patients in the intervention group received Jumpstart-Tips forms before the target visit. Study staff called subjects who had not yet returned their surveys after 3 to 4 weeks to ensure that the materials had been received, to answer any questions, and to send repeat mailings if necessary. Staff also offered to collect the survey data via telephone or in-person interviews, if the patient so desired. Questionnaire mailings included a $5 thank you that was not contingent on the receipt of the survey (survey research has shown that contingent incentives are ineffective).65
Withdrawals and Loss-to-Follow-up Data
Data on these events were collected similarly to refusals at recruitment: during telephone calls, before or after mailing questionnaires, or upon discovery of patient's death (through documentation in the EHR or in response to a phone call or mailing). Study staff entered these data into the tracking database.
Analytical and Statistical Approaches
Because all outcomes were specific to clinician–patient pairs, with each clinician potentially serving multiple patient participants, we based analyses on clustered regression models, with patients and family members clustered under clinicians. The predictor of interest for all models was the clinician's randomization allocation (intervention vs control), and all models included adjustment for the baseline measure of the outcome variable, if available, and for all confounders. We identified a variable as a confounder if its addition to the regression model that included only the intervention predictor altered the size of the coefficient for the intervention by more than 10%. Models with categorical outcomes were modeled with probit regression; those with continuous outcomes with linear regression; and those with censored outcomes with Tobit regression. We estimated linear regression models with restricted maximum likelihood, and probit and Tobit models with weighted mean- and variance-adjusted least squares (WLSMV). We define continuous (or pseudo-continuous) outcomes as censored if more than 25% of responses across all participants were at the minimum or maximum values on the response scale. We based analyses on intention to treat. We constructed the regression models detailed later in this document to test the intervention's effect on each outcome at that outcome's primary follow-up point. In addition, we compared the control and intervention groups' values on outcomes at other time points to provide insights into the intervention's durability of effect and to generate hypotheses for future studies. Finally, we investigated differences in perspective between clinicians and patient participants regarding whether goals-of-care discussions had occurred and conducted exploratory analyses of heterogeneity of treatment effects.
Analyses for Specific Aims
See Appendix F.
Subgroup Analyses and Heterogeneity of Treatment Effects
In addition to the regression models specified for the full sample, we ran exploratory stratified analyses within patient subgroups and tested for statistically significant interaction between the randomization group and the following potential effect modifiers: patient's baseline self-assessments of their general health status; their ratings of clinicians' quality of communication; and diagnosis with cancer, lung disease, or heart disease. These analyses are summarized in the Results section and details are in Appendix G.
Handling of Missing Data
The primary approach to missing data (either questionnaires or responses within a questionnaire) was to minimize the occurrence by reducing respondent burden, offering multiple methods for survey completion, and having trained staff in frequent contact with participants. We explored indirect tests of the mechanism of missingness by examining (1) baseline characteristics of those who ever vs never had missing data, by treatment group allocation, and (2) the difference between mean outcome scores of patients with missing data and those with complete data. To address missing data more directly, we used multiple-imputation (MI) models. The MI analyses included 83 subsample replications. Because these analyses produced results similar to those from the complete-case analyses, we have presented the complete-case analyses as the primary analyses in this report.
Avoidance of Bias
We minimized bias with the randomized controlled trial design, in which clinicians were randomly assigned to control or intervention. We also addressed the following potential sources of bias:
- Potential respondent bias in an unblinded study: The intervention precluded blinding clinicians, patients, or family members. This could introduce bias if patients or family members in the intervention arm gave different ratings for reasons other than the intervention (eg, to please researchers). However, participants in the 2 arms received the same amount and type of contact with study staff, which mitigated this potential source of bias.
- Nonparticipation and loss to follow-up: Patients, family members, and clinicians who agreed to participate may have been different from those who declined. Many patients or family members who refused to participate in this study might also refuse to participate in this intervention if it were introduced into clinical practice. This mitigates the effect that nonresponse bias has on the generalizability of the findings. We collected data on response rates and basic data on non-respondents (eg, age, race, gender, clinician specialty, patient diagnosis) to allow us to estimate the potential magnitude of bias from this source.
- Outcome assessment: We assessed the occurrence of goal-concordant care through patient reports and EHR documentation but did not record actual discussions. Although expert evaluation of recorded discussions might have provided additional insights into the occurrence and quality of goals-of-care discussions, it would have been extremely complex and prohibitively expensive to implement. However, patients' experience of these discussions and EHR documentation available to other clinicians are important components of this communication.
- Contamination: Because enrolled clinicians may work in clinics and on teams with other enrolled clinicians, it is theoretically possible that contamination through the teams could result in improved care delivered to the patients of clinicians in the control group. But this is unlikely to have a major effect, because the primary hypothesis and preliminary data suggest that care is unlikely to improve without the patient-specific feedback provided by the intervention. Because contamination would bias results toward the null hypothesis, the study results should provide a conservative estimate of an intervention effect.
Analyses of Qualitative Component
We deigned structured interviews to address 4 thematic areas: (1) Jumpstart-Tips strengths; (2) recommendations for Jumpstart-Tips improvements; (3) strengths of the study and procedures; and (4) recommendations for improvements to the study and procedures. The interviews were recorded, transcribed, and analyzed. Two study staff members reviewed the transcripts and completed a content analysis,66 sorting the responses into the 4 thematic areas and noting additional comments provided by the interviewees (eg, personal history of end-of-life decision-making). Trustworthiness was supported by reviewing findings with the investigators and the Community Advisory Board.
Changes to the Original Study Protocol
We made the following modifications to the funded application: (1) added another community study site; (2) changed sample size for patients, families, and IP team subjects based on analyses of baseline data and revision of the power calculations; (3) added a 6-month extension of the study to allow more complete collection of follow-up data; and (4) added a qualitative supplement that included interviews of patients, family members, and clinicians to explore the impact of the interventions and collect suggestions for improvements. These changes were submitted to and approved by the institutional review board, the Data Safety Monitoring Committee, and PCORI.
In 2014, the medical director of Providence Hospice approached us with an interest in conducting the study at Swedish Medical Center, a community-based teaching hospital in the Seattle area. We added this site to enhance patient recruitment and to extend the generalizability of our findings to systems outside UW Medicine. The revised budget with the SMC subcontract was approved by PCORI in September 2014. We obtained support from SMC's medical and administrative directors, submitted the study for institutional review, and began patient recruitment after our formal introduction and rollout to the primary clinicians at staff meetings in February 2015.
In December 2015, we formally proposed and submitted for approval the following modifications to the funded application: reduction in sample size for patient, family, and IP team subjects in response to a revision of sample size estimates based on preliminary baseline data from this study and in response to recruitment challenges; 6-month extension to the timeline to meet data collection and analysis needs; and addition of a qualitative research component to fully understand recruitment challenges, the impact of the intervention, and participants' perspectives on study outcomes, with the goal of enhancing future research. We changed the sample size for targeted enrollment of patients from 720 to 500. We did not identify specific sample size targets for family members and IP team clinicians, reflecting the difficulties of recruiting these 2 groups. These modifications were approved and implemented in March 2016. To address the loss of external validity associated with a reduced number of participating family members, we instituted the qualitative component to provide insights into family perspectives on the study in general and the Jumpstart-Tips form in particular.
The IRB for each hospital and clinic approved this study and the protocol modifications.
Results
Of 485 potentially eligible clinicians, we enrolled 132 (27% participation), with 65 randomized to intervention and 67 to usual care (Figure 1a). Of these 132 clinicians, 124 had patients participating in the study (3 clinicians changed practices and 5 had no patients enrolled). We identified 917 eligible patients, of whom 537 enrolled (59% participation), with 249 receiving the intervention and 288 receiving usual care (Figure 1b). Of these 537 patients, 494 contributed outcome data (23 became ineligible, 13 died, and 7 withdrew before the target clinic visit). Of 142 eligible family members identified by patient subjects for participation, 119 were enrolled into the study (84% participation) (Figure 1c). Four IP team members enrolled in the study. Given the small sample sizes for family and team members, analyses were not completed (team members) or were considered exploratory (family members). Clinicians were recruited between February 2014 and November 2015, and patients and family between March 2014 and June 2016.
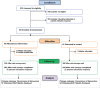
Figure 1a
CONSORT Flow Diagram - Clinicians.
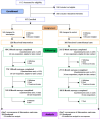
Figure 1b
CONSORT Flow Diagram - Patients.
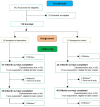
Figure 1c
CONSORT Flow Diagram - Family Members.
A slight majority of clinicians were women (53%), and their average age was 47.2 years (Table 1). A slight majority of patients were men (52%), and their average age was 73.5 years (Table 1). Most family members were women (75%), with an average age of 65.4 (Table 1). The most common chronic illness represented in the sample was advanced cancer (18%). Patients and family members were predominantly white non-Hispanic (79% and 84%, respectively), and 45% of patients reported fair-to-poor health status.
Table 1
Baseline Participant Characteristics of Clinicians, Patients, and Family Members.
Participating (n = 124) and nonparticipating (n = 361) clinicians did not differ significantly by racial/ethnic minority status, gender, age, or clinical degree (Table 2). However, participating and nonparticipating physicians differed significantly by specialty (P < .001). Participating (n = 494) and nonparticipating patients (n = 423) did not differ significantly by racial/ethnic minority status, gender, or age but were more likely to qualify for participation by virtue of having a high Charlson comorbidity score or being 90 years of age or older (Table 2).
Table 2
Differences Between Participants and Nonparticipants: Clinicians and Patients.
Aim 1
The Jumpstart-Tips intervention was associated with increased occurrence of goals-of-care discussions with clinicians at the target clinic visit and with patients' ratings of their clinicians' quality of communication at that visit (Table 3). Occurrence of such discussions was more likely in the intervention group among all patients (74% vs 31%; P < .001) and among the subset of patients who did not explicitly report at baseline that they wanted to avoid such a discussion (78% vs 28%; P < .001). Participating clinicians' EHR documentation of a goals-of-care discussion was also higher for the intervention group among all patients (62% vs 17%; P < .001), with similar findings for patients who did not explicitly report a desire to avoid discussion (63% vs 17%; P < .001). Quality ratings of clinicians' communication at the target visit were higher in the intervention group (mean values of 4.6 vs 2.1, P = .010, on the 4-indicator construct). Of the 7 items in the QOC_eol scale, the intervention group reported significantly higher ratings on 3 items (talking about patient's feelings about getting sicker, discussing patient's wishes for end-of-life treatments, and asking about things that are important to the patient). Differences between groups on the other 4 items were not statistically significant (Table 3).
Table 3
Effect of the Intervention on Occurrence and Quality of Patient–Clinician Communication About Advance Care Planning (Aim 1).
Aim 2
Three months after the target visit, patients' reports of their primary health care goal (comfort vs life extension) and the primary focus of their current care showed a nonsignificant difference in goal-concordant care (70% intervention vs 57% control; P = .077), with a greater between-group difference among patients whose goals were stable between the 3-month follow-up and the last previous assessment (73% intervention vs 57% control; P = .029; Figure 2).
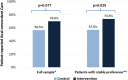
Figure 2
Percentage of Patients Reporting Goal-Concordant Care at 3 Months After Target Visit.
The other outcome of interest for this aim—use of palliative care services—was not significantly different by treatment group over the 6-month follow-up period for either the full sample or for those indicating a need for such a referral (Table 4). Only 40 patients died during the 6-month follow-up period; therefore, we did not analyze the association of the intervention with avoidance of life-sustaining treatments at the end of life.
Table 4
Effect of the Intervention on Provision of Care (Aim 2).
Aim 3
Patients: Patient symptoms of depression or anxiety at 3 and 6 months after the target visit did not vary significantly by treatment group as assessed by the standard composite scores or the 2-indicator latent variables (Table 5). In addition, we found no evidence of significant differences for any of the individual depression or anxiety items.
Table 5
Effect of the Intervention on Patients' Symptoms of Depression and Anxiety (Aim 3).
Families: Because of the small number of family members who participated in the study, we could not perform latent variable modeling to ascertain whether the standard PHQ-8 and GAD-7 composite measures exhibited unidimensionality and measurement invariance among treatment groups and over time. Therefore, the effects of the intervention on these measures at 3 and 6 months after the patient's target visit were tested without evidence that the outcomes were unidimensional. The scores had strong floor effects at all time points: 23.9% of family members scored 0 on the PHQ-8 score at baseline, 23.4% at 3 months, and 20.3% at 6 months. Comparable figures for the GAD-7 were 27.4%, 32.5%, and 36.5%. As a result, we declared the outcomes as censored from below and modeled them with Tobit regression. We adjusted each of the 4 models only for the respondent's baseline score on the outcome variable. In light of the small sample size, no testing was done for additional confounders. There were no treatment effects on any of the 4 outcomes.
Heterogeneity of Treatment Effects
We found no consistent evidence of heterogeneity of treatment effects for the outcomes by patient diagnosis (cancer vs no cancer, heart disease vs no heart disease, and lung disease vs no lung disease), patient self-reported health status (poor or fair vs good, very good, or excellent), or patients' baseline ratings of their clinician's communication (the latent 4-indicator QOC construct). (See Appendix G for more details.) Only 1 test that showed statistically significant interaction indicated a significant treatment effect in 1 of the strata being compared: In the cancer/noncancer comparison, the standard GAD-7 score at 3 months after the target visit yielded a significant treatment effect for patients with cancer (increased symptoms of anxiety in the intervention group compared with the control group) and a nonsignificant effect in the noncancer group.
Missing Data
To address missing data, we used multiple imputation models. We had complete data for the primary outcome for 80% of patients randomized, but 83% of the sample had missing data on 1 or more of the 51 outcomes and confounders we used in our analyses (41% failed to return at least 1 survey, and the remainder had 1 or more missing items on returned surveys). We repeated all analyses using MI, building 83 data sets for similarity to the percentage of cases with 1 or more missing data elements.67-69 The complete-case and MI analyses gave similar results, so only the complete-case analyses are shown in this report.
Qualitative Component
We interviewed 25 participants (10 patients, 5 family members, and 10 clinicians) regarding their participation in the study, the pros and cons of the Jumpstart-Tips form, and any recommendations they might have. The interviews were all transcribed and analyzed.
Patient interviews (n = 10) generally conveyed support for the Jumpstart-Tips form, though there were some criticisms. Positive reflections on the Jumpstart-Tips form included that it was helpful, highlighted important points, opened dialog, and made conversations easier with both clinicians and family members. Patients said the form helped them reach a comfort level with advance care planning (ACP) topics, introduced new ideas (extending life vs quality of life and CPR decisions), and was easy to understand and use. Some patients said they might use the form in the future and expected that their clinician might as well. Patients also expressed some criticisms. For example, some said the form's content was redundant and that it did not reflect care preferences that might change over time. The SUPPORT items required a choice that some respondents did not consider necessary; that is, they wanted to both extend life and enhance comfort, rather than selecting one or the other. Others (typically patients who had already had ACP conversations with their caregivers or clinicians before enrolling in the study) thought the intervention had little effect. Patients also made recommendations for improving the form, such as (1) readministering or reviewing it periodically to ensure that it still covers the patient's wishes, and (2) adding preferences about family involvement in care and decision- making. One participant suggested using a more subtle or lighthearted approach to advance care planning.
The family members (n = 5) we interviewed offered similar feedback about the Jumpstart-Tips form. Family participants thought the form was helpful, caused them to think more deeply about details, alleviated concerns, and clarified information. They also said that the form gave them a head start on ACP conversations; helped them feel more involved in the patient's care; and increased their awareness, expectations, and preparation. For those who had held previous ACP discussions, the form facilitated a reevaluation of their advance care plans. Family members' criticisms included the fact that they were trying to “stay positive” for the patient (with the implication that the study focus may have made that more difficult) and that the study was “one more thing to deal with.” Family members also provided some recommendations about the form, suggesting that it include a closing statement that “addresses the heart of all this” to acknowledge the difficulty of the task and reiterate the support of the health care team. Families wanted more information about the goal of the form and more specific information about the patient's disease. They also wanted the form to be tailored to the needs of a specific disease and to include information about whether the patient had completed a POLST form, a do not resuscitate order, or an advance directive.
Patients and family members also provided feedback about their overall perceptions of study participation, and most of these comments were positive. Respondents noted that they found participation to be beneficial even if somewhat difficult and that it helped raise important issues they would not have otherwise discussed, strengthened patient–family–clinician bonds, helped them feel like part of a team, prompted them to complete ACP paperwork, and increased their awareness about planning for the future. Participants also commented that it was helpful to go through the surveys. Some framed their participation as contributing to society, with participating patients noting that the study could benefit all by helping people understand their own roles as patients and empowering them to establish or regain control over their own care. Other participants felt that their participation could “provide information that might help other people on this difficult journey.” Participants also mentioned many concerns related to their participation—for example, (1) some participants felt it was too early for ACP discussions based on their perception of their illness or the current treatment plan; (2) some said that they felt uncomfortable and that participation was stressful; (3) some noted that they had already discussed these issues, making participation redundant; and (4) some said that the current system of multispecialty care by multiple providers inhibited effective ACP communication.
Participating clinicians (n = 10) were asked to reflect on their experiences with the Jumpstart-Tips form and the study in general. Most clinicians found the form helpful in various ways: (1) It was a good starting point/conversation starter/icebreaker; (2) it prompted patients to think about these issues beforehand so that they came into the visit prepared with ideas about ACP; (3) it structured the discussion, enabling the clinician to understand the patient's goals quickly and objectively; and (4) it provided a snapshot that helped keep ACP simple. In response to our request for recommendations, some clinicians noted that conversations before the target visit (such as those with the study coordinator during the baseline survey) may have been more influential than the form itself and that patients might have a better understanding of ACP because of these discussions. A few clinicians reported that some of their patients said their answers on the form no longer reflected their thoughts about their care—either their ideas had changed or they did not understand the concept (eg, “preferred vs perceived”). Clinicians suggested adding information on the Jumpstart-Tips form about whether the patient already has a POLST form and making the recommendations to the clinician less “wordy.”
Although a few clinicians raised questions about the timing of advance care planning and the awkwardness of an imposed conversation, the majority said that participation modestly enhanced the frequency and quality of interactions about advance directives with patients, both those in the study and those in their practices. Participation increased clinicians' awareness of the need for ACP and documentation and reminded clinicians not to assume knowledge of patient's wishes without actually discussing them with the patient.
Discussion
Context for Study Results
Prompting goals-of-care communication is a high priority in the care of patients with chronic life-limiting illness, because it offers opportunities for patients to identify their goals and enables clinicians and patients to jointly facilitate goal attainment.20,70,71 This patient-specific, communication-priming intervention was associated with increased goals-of-care communication during routine clinic visits between patients with serious illness and their primary and specialty care clinicians, whether the communication was measured by patient report or clinician documentation. In addition, the intervention was associated with higher patient ratings of the quality of the communication at the target visit, as assessed by patients 2 weeks after this visit. (We should note that a control group showed rates of goals-of-care discussion that were higher than those seen in previous studies, which suggests that the surveys alone, without the Jumpstart-Tips intervention, might have prompted communication.24,25)
Recent systematic reviews suggest that previous studies of communication tools were primarily designed to increase patients' use of advance directives and that these tools are associated with increased frequency of discussions about and completion of advance directives and with increases in goal-concordant care. However, the reviews assess the quality of the evidence presented by these studies as relatively low, owing to issues such as small sample sizes and observational designs.36-38 Our intervention differs from patient-facing decision aids or tools by obtaining specific information from patients to prompt and guide both patients and clinicians in a discussion about goals of care. This approach—simultaneously prompting both patients and clinicians—may have contributed to the large increase in the occurrence of communication, but our study design did not permit us to compare this approach to interventions that were solely patient-facing or solely clinician-facing. In addition, previous studies designed to educate clinicians to communicate about goals of care or advance care planning have suggested improved communication and improved patient outcomes, although the quality of evidence for improved patient outcomes has been relatively weak.26-30,72 Our intervention did not focus on clinician education (other than a brief 3-minute video on how to use the Jumpstart-Tips form). Future studies should consider combining a patient and clinician priming intervention such as the Jumpstart-Tips form with more robust clinician training in communication.
In this study, 23% of patients explicitly reported that they did not want to have a goals-of-care discussion. We examined between-group differences in goals-of-care discussions for all patients, as well as for those who did not explicitly report that they wanted to avoid such a discussion. Although patient-centered care might favor avoiding unwanted discussions, there can be value in raising these issues even with reluctant patients.73,74 The Jumpstart-Tips intervention alerted clinicians when patients were reluctant to discuss goals of care and equipped them with tailored recommendations to accommodate this reluctance (for example, taking an indirect approach to discussing prognosis).75
Our intervention was associated with an increase in patient-reported goal-concordant care at 3 months, although this finding was significant only for patients with goals that had remained stable since a previous point in time. The reduced treatment effect for the full sample may be attributable to the fact that patients who were uncertain about either their current goals or their current course of care were coded as receiving nonconcordant care. In a sense, the concept of concordance is inapplicable to patients whose goals are not stable. In addition, concordance was difficult to assess for patients whose goals vacillated over time, as the focus of their current care may have reflected goals that existed in the recent past but were no longer in effect. We believe that the analysis limited to patients whose goals appear to have been stable over time is a better representation of the intervention effect on goal-concordant care. This analysis included a group of patients whose treatment goals clinicians could be reasonably expected to infer from a discussion and for whom clinicians could design care that was in accordance with those goals. Further studies are needed to determine whether interventions to promote and improve goals-of-care discussions result in changes to delivery of end-of-life care. A previous intervention to promote goals-of-care communication for internal medicine trainees and nurse practitioner students was associated with an increase in depressive symptoms among patients.27 In the current study, we did not see evidence that facilitating these discussions among practicing clinicians was associated with an increase in symptoms of depression or anxiety. Although patients with cancer showed some evidence of increased symptoms of anxiety, these findings should be viewed as hypothesis-generating, given the number of comparisons in our analyses investigating heterogeneity of treatment effects. Future studies should continue to evaluate the effect of interventions to promote goals-of-care discussions on patients' psychological symptoms.
Previous studies have associated goals-of-care communication with improved patient and family outcomes, including increased satisfaction with care and reduced intensity of care at the end of life.9,15,16,76 Our study was not powered to investigate changes in end-of-life care, as only 40 patients died during the study. Further studies are needed to determine whether this intervention results in changes in end-of-life care.
In addition, we examined whether the intervention was associated with increased referral to palliative care services. The referral rates were very low and did not increase with the intervention. It may be that an intervention such as this one will not increase referral to palliative care, especially in the outpatient setting, until more palliative care specialists are available.
Implementation of this intervention into clinical practice would require creating or repurposing resources used to identify and survey eligible patients. The creation of Jumpstart-Tips forms could be automated from the algorithm we used (see Appendix C), and health care systems may want to adapt or update this algorithm using local palliative care expertise and norms. We have summarized other resources needed to implement this intervention (see Appendix H). However, whether the intervention would work as well outside a research study setting as it did in our study setting is not clear, and further evaluation is needed.
Generalizability of the Findings
The findings from this study are likely generalizable (with certain limitations) to patients with serious illness and clinicians caring for these patients. We believe that the Jumpstart-Tips intervention could be used in diverse outpatient settings and could potentially be used in the early stages of hospital admissions for seriously ill patients and their family members to facilitate goals-of-care discussions.
Implementation of Study Results
At a time when health care systems ask a lot of their clinicians in terms of tasks to complete and time management, incorporating another element of care for clinicians caring for patients with serious illness (and the patients' families) is challenging. We learned many important lessons about barriers during this study that will help us structure and guide implementation and uptake of this intervention. These lessons include the importance of engaging and accommodating busy clinicians who have many competing demands, explicitly addressing patients' reluctance to discuss goals of care, using sensitivity in approaching patients who are overwhelmed by illness and families that are overwhelmed with caregiving, providing a tool to help patients and family members have the goals-of-care conversations they want or need, and obtaining the support of the health care system and clinic leadership to implement the intervention.
Subpopulation Considerations
Among this group of patients with serious illness and a projected mortality of 2 to 3 years, we did not find consistent evidence of differences in the effectiveness of the intervention by patient diagnosis (cancer vs no cancer, heart disease vs no heart disease, and lung disease vs no lung disease); patient self-reported health status; or patients' baseline rating of clinicians' communication. Sample size limitations in these subgroups limited our ability to identify small but important differences in these subpopulations. Additional research is needed to examine whether other factors may differentiate responsiveness to this intervention and to identify populations that might benefit most from its implementation.
Study Limitations
This study has several limitations. First, although this was a multicenter study in diverse health care institutions, it took place in only 1 region of the United States and may not generalize to other regions. The population of the region is predominantly non-Hispanic white; additional studies are needed to evaluate the intervention in other groups and regions. Second, there may be selection bias among clinicians and patients who are willing to participate. We identified only a few variables associated with study participation, but differences may exist on unmeasured variables. Willingness to participate (especially among busy clinicians) may have been limited because this was a randomized trial in which the control arm received nothing beyond surveys; a phase IV evaluation of this intervention is needed to determine the barriers to implementation and dissemination. In addition, a clinician who is reluctant to participate might not receive the same benefit from the intervention. Third, measurement of goal-concordant care is a novel and challenging area of palliative care research that requires further study.21,77 Patient perceptions of both their goals and the focus of their care are important aspects in assessing goal-concordant care.21 Our approach may have been limited by patients' lack of clarity regarding their goals and their inability to discern the focus of their current care. Fourth, we recruited very few IP clinicians. We had hoped that IP clinicians might be able to support goals-of-care discussions, but we found that outpatient clinics are structured in a way that limits the number of IP clinicians available to play this role. Fifth, clinicians could be influenced by the intervention having been applied to a few patients of other clinicians in the same clinic (contamination), and we could not assess for this possibility in the current study. However, even if contamination occurred, it would bias the results toward the null hypothesis, making our estimates of benefit conservative. Sixth, we did not record discussions, which might have provided additional insights into the occurrence and quality of goals-of-care discussions. Finally, we did not assess previous communication training of clinicians, which could have influenced the effectiveness of the intervention.
Future Research
Our project reinforced the value of having engaged stakeholders from the start of a study. We found that patients with serious illness often were not well enough to participate in scheduled group events, but we had success with one-on-one interviews to obtain input from these patients. Future research will have to identify additional ways to engage patients with serious illness; for example, pursuing outreach via advocacy groups and employing flexibility in the approach to participation (eg, one-on-one interviews, alternative schedules and meeting locations).
We also developed a better understanding of barriers to recruitment of family members (eg, lack of time, uncertainty about the benefits of participation, patients protecting family members from stress of participation). More appropriate strategies are needed to attract members of this group. Ideas we heard from our Community Advisory Board and qualitative interviews include providing clearer justification and rationale for family participation, oversampling patients who have available families, and reducing the study burden (eg, time, questionnaires) for families.
On the basis of this feedback, we will continue to revise the study instruments for use in the future, including making them shorter and simplifying language. In addition, we will simplify the Jumpstart-Tips form for patients and shorten the form for clinicians. We will also consider using an “advance care planning facilitator” for patients who express a desire for conversation about ACP but for whom the form does not prompt discussion with their clinician. Additional and larger studies are needed to identify the best ways to implement this intervention and assess its effectiveness in routine practice in diverse clinical settings.
Conclusions
The principal aim of this research was to improve the methods health care systems use to provide patient-centered information to clinicians, with the specific goal of facilitating and enhancing communication about goals of care among patients with serious illness, their family members, and the clinicians who care for them. Our primary research question was the following: Does this patient-centered communication-priming intervention (Jumpstart-Tips) improve outcomes for patients with chronic, life-limiting illness and their families? Our results showed that the Jumpstart-Tips intervention was associated with an increase in patient reports and clinician documentation of goals-of-care communication between patients with serious illness and their primary and specialty care clinicians. The intervention was also associated with higher patient ratings of the quality of their clinician's communication and with increased patient-reported goal-concordant care among patients with stable goals. These findings suggest enhanced patient-centered care. There was no evidence that the intervention was associated with an increase in symptoms of depression or anxiety.
Although additional studies are needed to evaluate whether this communication is associated with changes in health care delivery, the use of a simple communication tool prepared from patient responses to a discrete set of questions and provided to clinicians before a routine clinic visit holds promise for health care systems that want to improve communication about goals of care for patients with serious illness.
The results of this study would be interesting to patients with serious illness, their family members, the clinicians who provide care for these patients, and health care systems that are interested in promoting and improving goals-of-care discussions between clinicians and patients with serious illness. The Jumpstart-Tips intervention could be implemented in an individual or group practice to promote goals-of-care discussions.
References
- 1.
- Abbott KH, Sago JG, Breen CM, Abernethy AP, Tulsky JA. Families looking back: one year after discussion of withdrawal or withholding of life-sustaining support. Crit Care Med. 2001;29(1):197-201. [PubMed: 11176185]
- 2.
- Hanson LC, Danis M, Garrett J. What is wrong with end-of-life care? opinions of bereaved family members. J Am Geriatr Soc. 1997;45(11):1339-1344. [PubMed: 9361659]
- 3.
- Zeytinoglu M. Talking it out: helping our patients live better while dying. Ann Intern Med. 2011;154(12):830-832. [PubMed: 21690597]
- 4.
- Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Understanding physicians' skills at providing end-of-life care: perspectives of patients, families, and health care workers. J Gen Intern Med. 2001;16(1):41-49. [PMC free article: PMC1495161] [PubMed: 11251749]
- 5.
- Fried TR, Bradley EH, O'Leary J. Prognosis communication in serious illness: perceptions of older patients, caregivers, and clinicians. J Am Geriatr Soc. 2003;51(10):1398-1403. [PubMed: 14511159]
- 6.
- Christakis NA, Lamont EB. Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320(7233):469-472. [PMC free article: PMC27288] [PubMed: 10678857]
- 7.
- White DB, Engelberg RA, Wenrich MD, Lo B, Curtis JR. Prognostication during physician– family discussions about limiting life support in intensive care units. Crit Care Med. 2007;35(2):442-448. [PubMed: 17205000]
- 8.
- White DB, Engelberg RA, Wenrich MD, Lo B, Curtis JR. The language of prognostication in intensive care units. Med Decis Making. 2010;30(1):76-83. [PMC free article: PMC2812635] [PubMed: 18753685]
- 9.
- Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. [PMC free article: PMC2844949] [PubMed: 20332506]
- 10.
- Tulsky JA, Chesney MA, Lo B. See one, do one, teach one? house staff experience discussing do-not-resuscitate orders. Arch Intern Med. 1996;156(12):1285-1289. [PubMed: 8651836]
- 11.
- Tulsky JA, Fischer GS, Rose MR, Arnold RM. Opening the black box: how do physicians communicate about advance directives? Ann Intern Med. 1998;129(6):441-449. [PubMed: 9735081]
- 12.
- Fischer GS, Tulsky JA, Rose MR, Siminoff LA, Arnold RM. Patient knowledge and physician predictions of treatment preferences after discussion of advance directives. J Gen Intern Med. 1998;13(7):447-454. [PMC free article: PMC1496983] [PubMed: 9686710]
- 13.
- Billings JA. The need for safeguards in advance care planning. J Gen Intern Med. 2012;27(5):595-600. [PMC free article: PMC3326115] [PubMed: 22237664]
- 14.
- White DB, Braddock CH III, Bereknyei S, Curtis JR. Toward shared decision making at the end of life in intensive care units: opportunities for improvement. Arch Intern Med. 2007;167(5):461-467. [PubMed: 17353493]
- 15.
- Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. [PMC free article: PMC2853806] [PubMed: 18840840]
- 16.
- Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169(5):480-488. [PMC free article: PMC2862687] [PubMed: 19273778]
- 17.
- Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. [PubMed: 23465068]
- 18.
- Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. [PubMed: 21133809]
- 19.
- Lo B, Quill T, Tulsky JA. Discussing palliative care with patients. ACP-ASIM End-of-Life Care Consensus Panel. American College of Physicians-American Society of Internal Medicine. Ann Intern Med. 1999;130(9):744-749. [PubMed: 10357694]
- 20.
- Tulsky JA, Beach MC, Butow PN, et al. A research agenda for communication between health care professionals and patients living with serious illness. JAMA Intern Med. 2017;177(9):1361-1366. [PubMed: 28672373]
- 21.
- Sanders JJ, Curtis JR, Tulsky JA. Achieving goal-concordant care: a conceptual model and approach to measuring serious illness communication and its impact. J Palliat Med. 2018;21(S2):S17-S27. [PMC free article: PMC5756461] [PubMed: 29091522]
- 22.
- Rietjens JAC, Sudore RL, Connolly M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol. 2017;18(9):e543-e551. [PubMed: 28884703]
- 23.
- Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153(4):256-261. [PMC free article: PMC2935810] [PubMed: 20713793]
- 24.
- Curtis JR, Engelberg RA, Nielsen EL, Au DH, Patrick DL. Patient–physician communication about end-of-life care for patients with severe COPD. Eur Respir J. 2004;24(2):200-205. [PubMed: 15332385]
- 25.
- Au DH, Udris EM, Engelberg RA, et al. A randomized trial to improve communication about end-of-life care among patients with COPD. Chest. 2012;141(3):726-735. [PMC free article: PMC3415164] [PubMed: 21940765]
- 26.
- Back AL, Arnold RM, Baile WF, et al. Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Arch Intern Med. 2007;167(5):453-460. [PubMed: 17353492]
- 27.
- Curtis JR, Back AL, Ford DW, et al. Effect of communication skills training for residents and nurse practitioners on quality of communication with patients with serious illness: a randomized trial. JAMA. 2013;310(21):2271-2281. [PMC free article: PMC4310457] [PubMed: 24302090]
- 28.
- Bernacki RE, Block SD; American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994-2003. [PubMed: 25330167]
- 29.
- Lakin JR, Koritsanszky LA, Cunningham R, et al. A systematic intervention to improve serious illness communication in primary care. Health Aff (Millwood). 2017;36(7):1258-1264. [PubMed: 28679813]
- 30.
- Chung HO, Oczkowski SJ, Hanvey L, Mbuagbaw L, You JJ. Educational interventions to train healthcare professionals in end-of-life communication: a systematic review and meta-analysis. BMC Med Educ. 2016;16:131. [PMC free article: PMC4850701] [PubMed: 27129790]
- 31.
- Sudore RL, Barnes DE, Le GM, et al. Improving advance care planning for English-speaking and Spanish-speaking older adults: study protocol for the PREPARE randomised controlled trial. BMJ Open. 2016;6(7):e011705. doi:10.1136/bmjopen-2016-011705 [PMC free article: PMC4947727] [PubMed: 27401363] [CrossRef]
- 32.
- Sudore RL, Boscardin J, Feuz MA, McMahan RD, Katen MT, Barnes DE. Effect of the PREPARE website vs an easy-to-read advance directive on advance care planning documentation and engagement among veterans: a randomized clinical trial. JAMA Intern Med. 2017;177(8):1102-1109. [PMC free article: PMC5710440] [PubMed: 28520838]
- 33.
- Volandes AE, Brandeis GH, Davis AD, et al. A randomized controlled trial of a goals-of-care video for elderly patients admitted to skilled nursing facilities. J Palliat Med. 2012;15(7):805-811. [PMC free article: PMC3387760] [PubMed: 22559905]
- 34.
- Volandes AE, Paasche-Orlow MK, Davis AD, Eubanks R, El-Jawahri A, Seitz R. Use of video decision aids to promote advance care planning in Hilo, Hawai'i. J Gen Intern Med. 2016;31(9):1035-1040. [PMC free article: PMC4978682] [PubMed: 27194151]
- 35.
- Volandes AE, Paasche-Orlow MK, Mitchell SL, et al. Randomized controlled trial of a video decision support tool for cardiopulmonary resuscitation decision making in advanced cancer. J Clin Oncol. 2013;31(3):380-386. [PMC free article: PMC4090424] [PubMed: 23233708]
- 36.
- Fawole OA, Dy SM, Wilson RF, et al. A systematic review of communication quality improvement interventions for patients with advanced and serious illness. J Gen Intern Med. 2013;28(4):570-577. [PMC free article: PMC3599019] [PubMed: 23099799]
- 37.
- Oczkowski SJ, Chung HO, Hanvey L, Mbuagbaw L, You JJ. Communication tools for end-of-life decision-making in ambulatory care settings: a systematic review and meta-analysis. PLoS One. 2016;11(4):e0150671. doi:10.1371/journal.pone.0150671 [PMC free article: PMC4847908] [PubMed: 27119571] [CrossRef]
- 38.
- Cardona-Morrell M, Benfatti-Olivato G, Jansen J, Turner RM, Fajardo-Pulido D, Hillman K. A systematic review of effectiveness of decision aids to assist older patients at the end of life. Patient Educ Couns. 2017;100(3):425-435. [PubMed: 27765378]
- 39.
- Teno JM, Gruneir A, Schwartz Z, Nanda A, Wetle T. Association between advance directives and quality of end-of-life care: a national study. J Am Geriatr Soc. 2007;55(2):189-194. [PubMed: 17302654]
- 40.
- Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362:1211-1218. [PMC free article: PMC2880881] [PubMed: 20357283]
- 41.
- Hammes BJ. What does it take to help adults successfully plan for future medical decisions? J Palliat Med. 2001;4(4):453-456. [PubMed: 11798476]
- 42.
- Hammes BJ, Rooney BL, Gundrum JD. A comparative, retrospective, observational study of the prevalence, availability, and specificity of advance care plans in a county that implemented an advance care planning microsystem. J Am Geriatr Soc. 2010;58(7):1249-1255. [PubMed: 20649688]
- 43.
- Wenrich MD, Curtis JR, Shannon SE, Carline JD, Ambrozy DM, Ramsey PG. Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Arch Intern Med. 2001;161(6):868-1674. [PubMed: 11268231]
- 44.
- Wenrich MD, Curtis JR, Ambrozy DA, Carline JD, Shannon SE, Ramsey PG. Dying patients' need for emotional support and personalized care from physicians: perspectives of patients with terminal illness, families, and health care providers. J Pain Symptom Manage. 2003;25(3):236-246. [PubMed: 12614958]
- 45.
- Dow LA, Matsuyama RK, Ramakrishnan V, et al. Paradoxes in advance care planning: the complex relationship of oncology patients, their physicians, and advance medical directives. J Clin Oncol. 2010;28(2):299-304. [PMC free article: PMC2815718] [PubMed: 19933909]
- 46.
- Lautrette A, Darmon M, Megarbane B, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356(5):469-478. [PubMed: 17267907]
- 47.
- Curtis JR, Treece PD, Nielsen EL, et al. Randomized trial of communication facilitators to reduce family distress and intensity of end-of-life care. Am J Respir Crit Care Med. 2016;193(2):154-162. PMID:26378963 [PMC free article: PMC4731711] [PubMed: 26378963]
- 48.
- Bandura A. Social Learning Theory. Prentice Hall; 1977.
- 49.
- Bandura A. Self-efficacy: toward a unifying theory of behavior change. Psychol Rev. 1977;84(2):191-215. [PubMed: 847061]
- 50.
- Bandura A. Self-efficacy mechanism in human agency. Am Psychol 1982;37(2):122-147.
- 51.
- Strecher VJ, DeVellis BE, Becker MH, Rosenstock IM. The role of self-efficacy in achieving health behavior change. Health Educ Q. 1986;13(1):73-91. [PubMed: 3957687]
- 52.
- Patrick DL, Grembowski DE, Durham M, et al. Cost and outcomes of Medicare reimbursement for HMO preventive services. Health Care Financ Rev. 1999;20(4):25-43. [PMC free article: PMC4194602] [PubMed: 11482123]
- 53.
- Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465. [PubMed: 10535437]
- 54.
- Cabana MD, Rushton JL, Rush AJ. Implementing practice guidelines for depression: applying a new framework to an old problem. Gen Hosp Psychiatry. 2002;24(1):35-42. [PubMed: 11814532]
- 55.
- Gerrity MS, DeVellis RF, Earp JA. Physicians' reactions to uncertainty in patient care: a new measure and new insights. Med Care. 1990;28(8):724-736. [PubMed: 2385142]
- 56.
- Glanz K, Rimer BK. Theory at a Glance: A Guide for Health Promotion Practice. National Institutes of Health, National Cancer Institute; 1997.
- 57.
- Billings ME, Curtis JR, Engelberg RA. Medicine residents' self-perceived competence in end-of-life care. Acad Med. 2009;84(11):1533-1539. [PMC free article: PMC5847268] [PubMed: 19858811]
- 58.
- Engelberg R, Downey L, Curtis JR. Psychometric characteristics of a quality of communication questionnaire assessing communication about end-of-life care. J Palliat Med. 2006;9(5):1086-1098. [PubMed: 17040146]
- 59.
- Teno JM, Fisher ES, Hamel MB, Coppola K, Dawson NV. Medical care inconsistent with patients' treatment goals: association with 1-year Medicare resource use and survival. J Am Geriatr Soc. 2002;50(3):496-500. [PubMed: 11943046]
- 60.
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. [PubMed: 16717171]
- 61.
- Lowe B, Decker O, Muller S, et al. Validation and standardization of the Generalized Anxiety Disorder screener (GAD-7) in the general population. Med Care. 2008;46(3):266-274. [PubMed: 18388841]
- 62.
- Martin A, Rief W, Klaiberg A, Braehler E. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28(1):71-77. [PubMed: 16377369]
- 63.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. [PubMed: 14583691]
- 64.
- Lowe B, Kroenke K, Grafe K. Detecting and monitoring depression with a two-item questionnaire (PHQ-2). J Psychosom Res. 2005;58(2):163-171. [PubMed: 15820844]
- 65.
- Dillman DA. Mail and Internet Surveys: The Tailored Design Method. John Wiley & Sons; 2000.
- 66.
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288. [PubMed: 16204405]
- 67.
- Bodner TE. What improves with increased missing data imputations? Struct Equ Modeling. 2008;15(4):651-675.
- 68.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. [PubMed: 21225900]
- 69.
- Allison, P. Why you probably need more imputations than you think. Published 2012. Accessed January 25, 2018. https:
//statisticalhorizons .com/more-imputations# :∼:text=Why %20do%20we%20need %20more,major%20reduction %20in%20this%20variability - 70.
- Meier DE, Back AL, Berman A, Block SD, Corrigan JM, Morrison RS. A national strategy for palliative care. Health Aff (Millwood). 2017;36(7):1265-1273. [PubMed: 28679814]
- 71.
- Institute of Medicine. Improving Quality and Honoring Individual Preferences Near the End of Life. National Academy Press; 2015. [PubMed: 25927121]
- 72.
- Bays A, Engelberg RA, Back AL, et al. Interprofessional communication skills training for serious illness: evaluation of small group, simulated patient interventions. J Palliat Med. 2014;17(2):159-166. [PMC free article: PMC4047839] [PubMed: 24180700]
- 73.
- Back AL, Arnold RM. Discussing prognosis: “how much do you want to know?” talking to patients who do not want information or who are ambivalent. J Clin Oncol. 2006;24(25):4214-4217. [PubMed: 16943540]
- 74.
- Jacobsen J, Brenner K, Greer JA, et al. When a patient is reluctant to talk about it: a dual framework to focus on living well and tolerate the possibility of dying. J Palliat Med. 2018;21(3):322-327. [PubMed: 28972862]
- 75.
- Curtis JR, Engelberg R, Young JP, et al. An approach to understanding the interaction of hope and desire for explicit prognostic information among individuals with severe chronic obstructive pulmonary disease or advanced cancer. J Palliat Med. 2008;11(4):610-620. [PubMed: 18454614]
- 76.
- Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292. [PMC free article: PMC4919118] [PubMed: 26784776]
- 77.
- Turnbull AE, Hartog CS. Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med. 2017;43(12):1847-1849. [PMC free article: PMC5717114] [PubMed: 28656453]
Related Publications
- •.
- Curtis JR, Downey L, Back AL, et al. A patient and clinician communication-priming intervention increases patient-reported goals-of-care discussions between patients with serious illness and clinicians: a randomized trial. JAMA Intern Med. 2018. Epub ahead of print. [PMC free article: PMC6145723] [PubMed: 29802770]
- •.
- Fakhri S, Engelberg RA, Downey L, et al. Factors affecting patients' preferences for and actual discussions about end-of-life care. J Pain Symptom Manage. 2016;52(3):386-394. [PMC free article: PMC5023466] [PubMed: 27265813]
Acknowledgments
We are grateful to the Cambia Palliative Care Center of Excellence Community Advisory Board for support and guidance in the conduct of this study. In particular, we acknowledge the leadership and support of the CAB cochairs, Priscilla Armstrong and Ronald C. Peck.
We are indebted to the patients, family members, and clinicians who participated in the study. Without their willingness and support, none of this research would have been possible.
We are also appreciative of the outstanding investigators and staff members who participated in this project. It was through their commitment, dedication, and excellent work that the project could be developed and completed.
Research reported in this report was [partially] funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (IH-12-11-4596). Further information available at: https://www.pcori.org/research-results/2013/improving-communication-preparing-patients-and-doctors-conversation-about-care-goals-serious-illness
Appendices
Appendix A.
Description of the Community Advisory Board (PDF, 210K)
Appendix B.
Examples of Jumpstart-Tips Forms (PDF, 326K)
Appendix C.
Algorithm for Creation of Jumpstart-Tips Form (PDF, 329K)
Appendix D.
Timeline of Data Collection for Outcomes (PDF, 225K)
Appendix E.
Outcome Instruments (PDF, 437K)
Appendix F.
Analytic Methods (PDF, 164K)
Appendix G.
Analyses for Heterogeneity of Treatment Effects (PDF, 908K)
Appendix H.
Resources Needed for Health Care System Implementation (PDF, 78K)
Suggested citation:
Curtis JR, Downey L, Back AL, et al. (2018). Improving Communication by Preparing Patients and Doctors for a Conversation about Care Goals for Serious Illness. Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/10.2018.IH.12114596
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Effect of a Patient and Clinician Communication-Priming Intervention on Patient-Reported Goals-of-Care Discussions Between Patients With Serious Illness and Clinicians: A Randomized Clinical Trial.[JAMA Intern Med. 2018]Effect of a Patient and Clinician Communication-Priming Intervention on Patient-Reported Goals-of-Care Discussions Between Patients With Serious Illness and Clinicians: A Randomized Clinical Trial.Curtis JR, Downey L, Back AL, Nielsen EL, Paul S, Lahdya AZ, Treece PD, Armstrong P, Peck R, Engelberg RA. JAMA Intern Med. 2018 Jul 1; 178(7):930-940.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Efficacy of a Communication-Priming Intervention on Documented Goals-of-Care Discussions in Hospitalized Patients With Serious Illness: A Randomized Clinical Trial.[JAMA Netw Open. 2022]Efficacy of a Communication-Priming Intervention on Documented Goals-of-Care Discussions in Hospitalized Patients With Serious Illness: A Randomized Clinical Trial.Lee RY, Kross EK, Downey L, Paul SR, Heywood J, Nielsen EL, Okimoto K, Brumback LC, Merel SE, Engelberg RA, et al. JAMA Netw Open. 2022 Apr 1; 5(4):e225088. Epub 2022 Apr 1.
- Review Interventions for interpersonal communication about end of life care between health practitioners and affected people.[Cochrane Database Syst Rev. 2022]Review Interventions for interpersonal communication about end of life care between health practitioners and affected people.Ryan RE, Connolly M, Bradford NK, Henderson S, Herbert A, Schonfeld L, Young J, Bothroyd JI, Henderson A. Cochrane Database Syst Rev. 2022 Jul 8; 7(7):CD013116. Epub 2022 Jul 8.
- Review Examining Home Visits from Community Health Workers to Help Patients Manage Asthma Symptoms[ 2021]Review Examining Home Visits from Community Health Workers to Help Patients Manage Asthma SymptomsStout J, Chen R, Farquhar S, Kramer B, Song L. 2021 Dec
- Improving Communication by Preparing Patients and Doctors for a Conversation abo...Improving Communication by Preparing Patients and Doctors for a Conversation about Care Goals for Serious Illness
Your browsing activity is empty.
Activity recording is turned off.
See more...
