NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Committee on the Public Health Dimensions of Cognitive Aging; Board on Health Sciences Policy; Institute of Medicine; Blazer DG, Yaffe K, Liverman CT, editors. Cognitive Aging: Progress in Understanding and Opportunities for Action. Washington (DC): National Academies Press (US); 2015 Jul 21.

Cognitive Aging: Progress in Understanding and Opportunities for Action.
Show detailsThe brain is subject to a lifetime of demands and exposures, both beneficial and deleterious. Given the importance to the public's health of preventing individuals' cognitive impairment and promoting their cognitive health, it is important to develop an in-depth understanding of these various beneficial and deleterious factors to guide prevention and remediation efforts. Risk and protective factors include all of the personal lifestyle, behavioral, social, medical, and genetic characteristics of an individual or environment that are associated with, respectively, decreases or increases in cognitive function. There is wide variability in cognitive functioning levels among individuals, and risk and protective factors may affect a person's long-term cognitive trajectory in varying ways. Most of the interventions that have been developed to date focus on prevention efforts, although some remediation efforts are also being explored.
This and the next two chapters explore risk and protective factors and interventions organized into discussions of lifestyle factors and the physical environment (Chapter 4A), medical and physical factors (Chapter 4B), and general approaches to remediation (Chapter 4C). Details of the organization are provided in Table 4A-1. This chapter begins with an overview of some of the opportunities and challenges associated with examining the risk and protective factors for cognitive aging and determining the effectiveness of interventions. Subsequent sections in Chapters 4A and 4B focus on specific risk or protective factors. Each discussion provides an overview of observational and intervention studies, followed by a summary comment on the strength of the evidence. Chapter 4C discusses interventions that are not specific to any one risk factor, and it also provides the committee's recommendations on actions needed to better prevent, delay, or attenuate cognitive aging.
TABLE 4A-1
Risk and Protective Factors and Interventions Discussed in the Report.
ISSUES IN STUDYING RISK AND PROTECTIVE FACTORS AND INTERVENTIONS
This report uses a health promotion approach because such an approach is applicable to broad community-based populations and also applicable across the life span. Efforts to promote cognitive health should begin early to have maximal benefit. Discussions about prevention become complex because cognitive aging is not a disease but rather is a highly variable process of cognitive change as individuals get older.
Further complicating discussions of risk and protective factors for cognitive aging is the intertwined and not fully understood nature of the relationship between medical conditions that may result in short-term cognitive decline (e.g., stroke, delirium, and medications that compromise cognition) and cognitive aging over time. Although some individuals with acute decline may return to their baseline level of cognitive functioning, others may make only a limited recovery, and still others may find that their cognitive function does not improve at all; the long-term impacts of acute cognitive decline on cognitive aging are not fully understood. Furthermore, much of the literature on medical-related risk factors has focused on neurodegenerative diseases and less research on these factors has been devoted to cognitive aging per se.
One of the overriding issues in assessing the role of risk factors as correlates or determinants of cognitive aging is the socioeconomic status (SES) of the individual or the group of interest (Deary, 2012). SES is often measured by an individual's social status, educational attainment, occupational level, and income. Importantly, it is one of the strongest correlates of cognitive status, performance, and outcomes, and these associations persist across the life span (Kuh et al., 2014). SES is also correlated with many other health states and outcomes, including those related to infant and childhood developmental outcomes, and thus it is an important consideration—both as a determinant and a confounder of associations—in any analysis and interpretation of cognitive aging studies of risk and intervention programs (Wong and Edwards, 2013). In many instances, SES is likely to be a surrogate for various environmental and genetic exposures, which, if possible, should be identified.
Because of the limited number of available studies of any type on cognitive aging, the committee decided to include the wide range of available studies but also to note the challenges and limitations of the studies where relevant. Although often the available studies evaluate cognitive outcomes with only short-term follow-up and do not examine long-term cognitive aging trajectories, the committee determined that since the short-term cognitive effects are potentially preventable, they should be noted in this report.
In compiling the evidence for each of the relevant factors and interventions, the committee did not conduct new systematic reviews of primary evidence. It based its assessments on existing systematic reviews, updated literature reviews, original articles, clinical guidelines, and input from experts. The goal was to create a synthesis of the evidence that would be sufficient to guide recommendations on important risk factors and on the interventions to address them. While this report's focus is on cognitive aging, some of the included studies addressed the outcomes of cognitive impairment, dementia, and Alzheimer's disease.
One major effort to evaluate recent studies that have examined modifiable risk factors for cognition and dementia reviewed 247 studies published between January 1990 and October 2012, each of which included at least 300 generally healthy people and used either a cross-sectional or a prospective (longitudinal) study design (Beydoun et al., 2014a). The authors considered all cognitive outcomes, including global and domain-specific cognitive outcomes, cognitive decline, prevalent and incident mild cognitive impairment (MCI), dementia, and Alzheimer's disease. A variety of modifiable risk factors was considered, including education, physical activity, and nutrition. The committee drew on this comprehensive review when summarizing the available evidence on modifiable risk factors, but also examined other reviews pertinent to individual risk factors as well as primary studies. The committee recognized a number of methodological challenges, which are described in subsequent sections.
Interpreting Observational Studies
Observational studies are designed to collect data from people regarding their risk factors and cognitive abilities without experimenting upon them by, for example, giving them either an active intervention (such as a pill or exercise) or a placebo. While hundreds of observational studies have been published on risk factors for cognitive decline and dementia or Alzheimer's disease, it is often difficult to draw firm conclusions from them. The studies differ in many ways, having various strengths and limitations, and their findings are often inconsistent. The following factors affect the interpretation of the evidence about the risk factors for cognitive aging and the ability to draw conclusions from that evidence:
- Age range: Ideally, an evaluation of risk factors for cognitive aging would include representative populations of adults followed from young adulthood to very late life. Many studies include only cross-sectional (one-time) examinations or short follow-up periods, and their conclusions are inherently limited.
- Measures of cognitive changes: As noted in Chapter 2, there are many challenges in measuring cognitive change with age, including the wide variety and large number of available assessment measures. Instruments are needed that eliminate or minimize cultural or race/ethnicity bias. Methods that account for practice effects are important to utilize. Because baseline information is critical and the focus is on change across time in varying cognitive domains (see Chapter 2), it is useful to have multiple measurements conducted at various points across the life span in order to more fully inform conclusions on risk and protective factors.
- Study outcomes: Study outcomes need to measure effects on cognitive aging and outcomes other than the development of diseases. Cognitive aging can occur in the adult population without the eventual development of disease. In many existing studies, cognitive outcomes are often combined (e.g., both cognitive decline and dementia) or poorly specified. For this report, cognitive decline was the most relevant study outcome; however, when no studies reporting cognitive decline were available, the committee chose to use dementia outcomes (in some sections) with full recognition of the limitations of this approach.
- Confounding: Sometimes risk and protective factors may be associated with cognitive outcomes because of the influence of other factors that have not been or that cannot be accounted for in the study. Such “confounding” is a major impediment to determining whether a causal association exists between a risk factor and a cognitive outcome.
Because of these issues, it is difficult to firmly establish in many cases whether a risk factor is causing cognitive changes with age or whether changing the risk factor would alter the course of cognitive function. Despite these limitations, the overviews of observational studies provide the best currently available evidence on risk factors that may be modifiable and that have been studied sufficiently to help produce reasonable recommendations on risk factors now; these overviews of observational studies also point to important avenues for future research. The committee acknowledges that the list of risk and protective factors is not exhaustive; rather, it is heavily weighted toward factors that are reasonably common, are potentially preventable, and have a substantial evidence base.
Interpreting Intervention Studies
Intervention studies have the potential to provide strong evidence for behavior change or other interventions that could mitigate the risk factors associated with cognitive aging. The synergistic effects of various components of an intervention (e.g., the social component of a physical activity intervention) also add to the opportunities and the challenges of understanding cognitive aging. As discussed in Chapter 4C, a number of intervention studies are examining the effects of multicomponent interventions.
The evidence base for cognitive aging–related interventions has many gaps, some of which are noted here. First, for many interventions, well-designed rigorous studies, including randomized controlled trials (RCTs), have not been conducted. In some situations where interventions are known to be beneficial, a true control (i.e., non-intervention group) is often not ethical.
Second, studies have not used consistent outcome measures. Because each specific task or measure is subject to measurement error, cognitive change should be assessed using several tasks or measures in order to reduce error variance and converge on important constructs. Some studies have used multidimensional measures of cognition or dementia (e.g., the Mini-Mental State Examination [MMSE]), whereas others have examined only specific tasks (e.g., the digit–symbol substitution test), and still others have used structural or functional brain changes (e.g., total brain or hippocampal volume, or activation of brain regions as cognitive tasks are performed) as proxies for cognitive decline. As a result, the clinical meaning of changes associated with particular interventions is still uncertain.
Moreover, the time frames for administration of interventions and for the assessment of their effects vary widely. Some interventions have looked at short-term outcomes, whereas others have looked for changes decades after the intervention. A problem shared by intervention trials and prospective observational studies is that individuals often improve at taking cognitive tests over time because of “learning effects,” which can mask declines in cognition. Furthermore, it is unclear whether cognitive interventions translate into real-life behavior changes that can be sustained over time.
Additionally, much remains to be learned about the biological mechanisms by which a given intervention affects cognition and also the psychological factors (e.g., control beliefs, changes in self-efficacy, or the acquisition of cognitive strategies) by which positive change can be sustained.
Finally, there are various costs of some interventions aimed at improving cognition that must be taken into account, including costs in time, money, and even the safety of the individual. Although some lifestyle changes (e.g., physical activity) may have significant benefits beyond cognition, others (e.g., transcranial direct current stimulation) have the potential for harm, even when no such harm has not been identified in short-term studies. The monetary cost of some interventions (e.g., dietary supplements) also must be considered. Indeed, the diversion of resources to pay for ineffective rather than effective treatments is an important public health consideration.
PHYSICAL ACTIVITY AND EXERCISE
Physical activity is strongly linked to healthy aging and remaining independent, and it has been associated with helping individuals maintain their physical and cognitive function throughout life and also with older adults developing fewer chronic conditions (Lee at el., 2012; NIH, 2014b). Conversely, ample evidence suggests that low levels of physical activity are associated with an increased risk of developing a number of diseases including stroke, hypertension, type 2 diabetes, osteoporosis, and a variety of cancers including colon and breast cancer (Lee et al., 2012).
Evidence from Observational Studies
The relationship between physical activity and cognitive heath has been studied over decades. In the 1970s Spirduso and colleagues compared the performance of younger and older individuals, both athletes and non-athletes, on a series of reaction time and movement tasks (Spirduso, 1975; Spirduso and Clifford, 1978) and observed that older athletes outperformed older non-athletes on many of these tasks. These results, and others, led to a multitude of cross-sectional studies that generally found older high-fit individuals to perform better than low-fit adults on a number of perceptual, cognitive, and motor tasks (Abourezk and Toole, 1995; Clarkson-Smith and Hartley, 1990). Longitudinal observational studies have also generally found a relationship between physical activity and cognitive health outcomes. For example, Zhu and colleagues (2014) found that a graded exercise measure of cardiorespiratory fitness predicted performance on a variety of cognitive tasks 25 years after the original measurement, even after accounting for differences in participants' race, sex, age, and education.
Several recently published systematic reviews (Anderson et al., 2014; Beydoun et al., 2014b; Blondell et al., 2014), as well as many qualitative reviews (Ahlskog et al., 2011; Bherer et al., 2013; Bielak, 2010; Lautenschlager et al., 2012), have reiterated these findings. A 2014 review included 21 prospective studies on physical activity and cognitive decline and 26 prospective studies on physical activity and dementia (Blondell et al., 2014). A meta-analysis was conducted on these studies, and as shown in Figure 4A-1, the majority showed benefit, with the overall finding that physically active adults had a 35 percent lower risk of cognitive decline (relative risk [RR] = 0.65; 95% confidence interval [CI] 0.55–0.76) than those who were inactive.
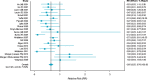
FIGURE 4A-1
Studies on physical activity and cognitive decline. SOURCE: Blondell et al., 2014.
Studies with the highest-quality, longest follow-ups, and with a greater number of adjustments for differences between study designs, have yielded findings about the value of exercise that are more conservative but still in the protective direction (Blondell et al., 2014). In the Study of Osteoporotic Fracture, women who reported more physical activity at any time in their life course (adolescence and at age 30 years, 50 years, or in later life) had lower risk of cognitive impairment than inactive women; the association was especially strong for women who had been active teenagers (Middleton et al., 2010). In a recent systematic review, 21 of 24 prospective studies found an association between physical activity and cognitive outcomes, and all four of the cross-sectional reports included did so as well (Beydoun et al., 2014b).
Evidence from Intervention Studies
A substantial number of intervention studies have reported that physical activity and exercise have a benefit on cognition. For example, in one early randomized trial, three groups of older adults (an aerobic exercise group, a strength and flexibility group, and a non-exercising control group) were tested on a variety of cognitive tasks before and after the 4-month intervention. The aerobic exercise group demonstrated performance improvements on a number of cognitive tasks1 (Dustman et al., 1984). In an effort to examine the specificity of exercise's effects on cognition, Kramer and colleagues (1999) conducted a 6-month intervention trial with 124 older adults. Subjects were randomized to two groups, a walking group (aerobic) and a toning and stretching (non-aerobic) control group. The primary questions addressed were whether performance on tasks that had components of executive control processes would be improved for the walkers but not for the stretching group and whether non–executive control processes would show equivalent improvements for both exercise groups. The walking group improved to a significantly greater extent than the toning and stretching group in skills that involve aspects of executive brain function. Other interventions have often, but not always, found aerobic exercise interventions to have beneficial effects on cognition (Blumenthal et al., 1991; Madden et al., 1989).
A series of meta-analyses (Angevaren et al., 2008; Colcombe and Kramer, 2003; Smith et al., 2010b) has helped to clarify this literature. In general, these meta-analyses have found modest effect sizes for the exercise–cognition relationship. Some of the meta-analyses have also examined the moderating effects of various factors. For example, Colcombe and Kramer (2003) found that the relationship between exercise and cognition had larger benefits for executive tasks than for other cognitive processes (but also had benefits across different dimensions of cognition). They also found the exercise–cognition relationship to be influenced by gender, intervention duration, age, and exercise type. Women showed larger benefits than men. Combined aerobic and strength programs showed greater benefits than aerobic programs alone. People older than 65 years showed more benefits than those from 55 to 65 years of age, and exercise sessions longer than 30 minutes showed larger cognitive benefits than shorter ones.
More recently, behavioral assessments of physical activity and exercise effects have been augmented with human neuroimaging studies, particularly studies using functional magnetic resonance imaging (fMRI). To briefly summarize this expanding literature, intervention studies have found that increases in aerobic fitness are associated with increased volume in a number of brain regions—in particular, the frontal and hippocampal areas, which generally show a decrease in volume during adult aging (Colcombe et al., 2006; Erickson et al., 2010). However, other studies have found structural changes in the brain to be associated with increased physical activity, regardless of exercise type (both aerobic and non-aerobic activity) (Ruscheweyh et al., 2011; Voss et al., 2013a). Furthermore, studies using fMRI have found that as participants' aerobic fitness improves, there are changes in certain patterns of brain activation that are known to predict increased performance on executive function and processing speed tasks (Colcombe et al., 2004; Rosano et al., 2010). The fMRI studies have also found increased functional connectivity among brain regions that are known to contribute to better executive function and memory after 4 to 12 months of aerobic exercise interventions (Burdette et al., 2010; Voss et al., 2010).
Animal research conducted over the past couple of decades complements these studies by revealing the underlying molecular and cellular mechanisms that are associated with exercise-related improvements in cognition. This literature is extensive and several thorough reviews can be consulted for details (Cotman and Berchtold, 2002; Vaynman and Gomez-Pinilla, 2005; Voss et al., 2013b).
Summary
A consensus is emerging in the scientific community that physical activity can slow or attenuate cognitive decline and improve cognitive function in middle-aged and older adults. Possible mechanisms include indirect effects, such as aerobic exercise optimizing vascular health throughout life and reducing the burden of cerebrovascular disease, as well as direct effects on slowing neurodegenerative processes and improving the body's own neuroprotective neurotrophic factors and neuroplasticity (Ahlskog et al., 2011). This increasing body of literature has prompted several panels to recommend minimum levels of physical activity on a daily and weekly basis (Haskell et al., 2007; HHS, 2014a,b). Although the evidence is strong that physical activity and exercise can positively affect cognitive aging, many unanswered questions need to be addressed in future research, such as:
- Do different modes of exercise produce different cognitive benefits?
- Can different exercise parameters (e.g., intensity, duration, interaction with other lifestyle factors) optimize cognition and brain health benefits?
- To what extent do genetic differences, particularly in genes related to cognition, moderate the effects of exercise on cognition?
- Is there an optimal period of life in which to begin exercise to reap the largest cognitive benefits?
- Are the effects of exercise synergistic with other lifestyle factors?
- How best can individuals be helped to start and sustain appropriate regular physical activity throughout their lives?
EDUCATION AND INTELLECTUAL ENGAGEMENT
Educational attainment is a well-known demographic characteristic that is correlated with peak cognitive ability, and inversely correlated with cognitive decline and dementias, including Alzheimer's disease. Low literacy and low educational attainment often reflect, in part, adverse early life conditions that may have deleterious effects on cognitive abilities throughout life. Educational attainment is one of the easiest and most consistently evaluated measures of SES. However, the challenge with studies on education and cognitive aging is clarifying the cause and effect (EClipSE Collaborative Members et al., 2010). Many midlife and older adults are involved in a wide variety of intellectual activities that extend beyond traditional education, including taking college or enrichment courses, reading, learning new languages or musical instruments, and doing crossword puzzles or other types of efforts to learn more or challenge themselves. The extent and impact of these efforts on cognitive aging is not fully known (ACE and MetLife, 2007).
Higher levels of education may allow older people to perform better on cognitive tests (Meng and D'Arcy, 2012) and may provide a buffer against cognitive decline. This finding is consistent with the concept of “cognitive reserve.” As noted in Chapter 2, cognitive reserve is a theoretical concept proposing that certain lifetime experiences, including education, degree of literacy, and occupational attainment, increase the flexibility, efficiency, and capacity of brain networks, thereby allowing individuals with higher cognitive reserve to sustain greater levels of brain pathology before showing clinical impairment (for a review of cognitive reserve, see Stern, 2009). Supporting the concept of cognitive reserve, cross-sectional studies of nondemented and demented individuals have reported greater levels of brain atrophy among individuals with higher cognitive reserve than among those with lower cognitive reserve and similar levels of cognitive functioning, suggesting that the effects of atrophy on cognition are reduced in individuals with higher reserve (Arenaza-Urquijo et al., 2013; Liu et al., 2012; Querbes et al., 2009; Reed et al., 2010; Sole-Padulles et al., 2009). In older adults without dementia, studies suggest that education is strongly associated with cognitive performance globally, as well as in many specific domains, but that it is not associated with the rate of cognitive decline (Singh-Manoux et al., 2011; Zahodne et al., 2011). Thus, cognitive reserve appears to delay the onset of the clinical signs of dementia, including Alzheimer's disease, while in cognitively healthy people, it is associated with higher cognitive performance throughout life but no difference in the rate of cognitive decline with aging.
Cross-sectional studies of older adults have also shown positive relationships between literacy and cognitive performance even after adjusting for level of education (Albert and Teresi, 1999; Federman et al., 2009; Jefferson et al., 2011). Two longitudinal studies evaluated the association between literacy and cognitive decline in aging and found that, even after controlling for education, individuals with lower literacy showed greater declines in memory, language, and executive functioning (Manly et al., 2003, 2005). Furthermore, literacy has been shown to help explain difference between black and white older adults in both cognitive performance and rates of cognitive decline, again after controlling for education (Mehta et al., 2004; Sachs-Ericsson and Blazer, 2005).
More highly educated people also typically have lower risk of cardiovascular disease and engage in healthier behaviors; these differences can translate into more favorable cognitive trajectories during adulthood (Hendrie et al., 2006). However, race/ethnicity, country of origin, cultural diversity, and language fluency also relate to educational attainment and the ability to score well on cognitive tests, making it extremely difficult to disentangle the influence of these various factors. In a systematic review, 18 of 27 prospective studies and 21 out of 25 cross-sectional studies found that lower educational attainment was associated with worse cognitive outcomes (Beydoun et al., 2014a).
Some studies have addressed the impact of intellectual engagement and lifelong learning on cognitive outcomes. Such studies often record individuals' self-report of intellectually challenging activities at one point in time and compare the number and frequency of these activities with measures of cognition or the diagnosis of neurodegenerative disorders years later. In a study of 294 older adults, Wilson and colleagues (2013) found that greater cognitive activity in early life (e.g., reading books, visiting a library, writing letters, etc.) was associated with a lower rate of cognitive decline later in life. This relationship (increased activity early in life associated with decreased rate of cognitive decline at a later time) remained when cognitive declines associated with neuropathology were removed from the equation. These results might be viewed as an example of cognitive reserve—that is, an enhancement of cognition either earlier in life or in late life that serves as a protective factor with aging (Stern, 2012; see Chapter 2). In examining data on intellectual engagement and lifelong learning efforts (midlife and later life) in participants of the Mayo Clinic Study of Aging (a longitudinal population-based study of cognitive aging in Olmsted County, Minnesota), researchers found that intellectually related efforts may have protective effects on cognitive aging (Geda et al., 2011; Vemuri et al., 2014). There are also ongoing studies comparing cognitive training and cognitive engagement activities (Stine-Morrow et al., 2014). The challenges of studies in this area include the many different types of activities that individuals engage in, the varying extent to which these activities are engaged in by individuals, and the long-term nature of follow-up. Many of these studies are observational, and while they are important in establishing the relationship between cognitively stimulating activities and cognitive decline or age-related neurodegenerative diseases, they do not establish causality.
Summary
Education is one of the most consistent and influential personal characteristics influencing cognitive function throughout life (Beydoun et al., 2014a; Hendrie et al., 2006). Much remains to be learned about the extent to which there is a cognitive reserve and, if so, what are its physiological mechanisms. As educational levels become more uniform in advanced societies, indicators of the quality of education, especially literacy, may become better predictors of cognitive function. The available evidence emphasizes the importance of both early life education and maintaining cognitive function over the life span for peak cognitive capacities. Research is also showing that intellectual engagement and lifelong learning are associated with positive cognitive outcomes, although there is still much to be learned regarding the specific activities, duration of efforts, and other factors that influence cognitive outcomes.
SOCIAL ISOLATION, LONELINESS, AND SOCIAL ENGAGEMENT
Evidence from Observational Studies
Social Isolation and Loneliness
Loneliness can be defined as the emotional state caused by unfulfilled social and intimacy needs (Luanaigh and Lawlor, 2008). The concept of loneliness is distinct from being alone, or social isolation, which is usually defined as living alone, not having a partner, and having few social supports (Steptoe et al., 2013). The perception of loneliness is strongly related to depression (Luanaigh and Lawlor, 2008). Cacioppo and Hawkley (2009) have described a number of possible mechanisms connecting loneliness with decline in cognition, including (1) stress and activation of the hypothalamic–pituitary–adrenal axis with possible increased inflammatory responses in the brain; (2) less neuroplasticity in those older individuals who lack interaction with others, resulting in less ability to compensate for age-related neurodegenerative changes; (3) lower cognitive stimulation because of social isolation; (4) increased cognitive demand due to chronic surveillance for and protection from threats; (5) increased depression and decreased physical activity; (6) reduced quality and quantity of social interactions; and (7) diminished sleep quality resulting in impaired learning.
Several studies have shown that social isolation and loneliness are associated with declines in global cognition, psychomotor processing speed, and delayed visual memory (O'Luanaigh et al., 2012); with cognitive decline (Shankar et al., 2013; Tilvis et al., 2004); Alzheimer's disease (Holwerda et al., 2014; Wilson et al., 2007); and other physical problems, including sleep disruption, blood pressure, inflammation, and heart disease. Some evidence suggests that loneliness is more detrimental than social isolation (Holwerda et al., 2014) and that poorly educated people are affected more than those who are more educated (Shankar et al., 2013). Cognitive declines may lead to lower social engagement, creating a downward spiral of social isolation and loneliness.
Social Engagement
A growing and diverse body of evidence has evaluated whether social engagement, social activities, social support, and social strain are related to cognitive decline with age. The measures of social activity in this research vary widely, with some including lifestyle activities that require both physical and cognitive effort. One example is the Life Complexity Inventory used originally in the Seattle Longitudinal Study (Schaie, 1996). This scale is constructed by combining scores for the frequency of various activities, including going to parties, volunteering, going to dances, playing cards, visiting others, attending church, and speaking with friends and family on the phone (Brown et al., 2012). Despite the diversity of measures used in the various studies, some consistent patterns are emerging. First, social activity, including social contacts and social support, is usually associated with improved cognitive abilities at baseline or at any single point in time (Barnes et al., 2013; Bielak, 2010; Brown et al., 2012; Seeman et al., 2011). However, some studies have found that higher cognitive measures predicted greater social engagement (Bielak, 2010; Brown et al., 2012). This leaves open the possibility that people with higher cognitive abilities choose to be more socially engaged, on average, than those with lower cognitive abilities (Gow et al., 2012). Second, social engagement is generally related to some cognitive domains—including speed of processing and various aspects of memory—but not to all (Gow et al., 2012). Third, increases in social activity are associated with higher levels of cognition, at least in some studies (Brown et al., 2012; Carlson et al., 2009; Stine-Morrow et al., 2008). Fourth, there can be negative associations between social engagement and cognitive outcomes, particularly when those activities cause social strain or stress (Seeman et al., 2011; Tun et al., 2013). For example, a study of grandparenting by postmenopausal women found that working memory and processing speed were positively associated with spending time with grandchildren 1 day per week, while poorer cognition was associated with minding grandchildren several days per week, a situation that prompted feelings of greater demand (Burn et al., 2014). Fifth, there may be gender differences in associations between social engagement and cognitive aging. For women, there is evidence that social engagement relates to better cognitive abilities (Thomas, 2011). The phenomenon of older adults with cognitive declines relinquishing social engagement has also been observed in studies that did not report results by gender (e.g., Small et al., 2012). This large and evolving evidence base lends support to the value of social activity for maintaining cognitive health and suggests the importance of using many possible approaches to increasing social engagement.
Evidence from Intervention Studies
Very few intervention studies have examined the effects of socialization alone on cognition. The majority of these have been small pilot studies (e.g., Carlson et al., 2009) involving people with dementia or else they have been multimodal—that is, they combined socialization with physical activity or cognitive stimulation (e.g., Kamegaya et al., 2014). One such study did find a small improvement from a social interaction intervention as compared with other activities or no intervention. A study conducted in Shanghai by Mortimer and colleagues (2012) randomized adults 60 to 79 years of age (40 men and 80 women) without dementia to one of four groups (tai chi, social interaction, walking, or no interaction). The socialization group met with a group leader and assistant for 1 hour three times per week at the local community center. Cognitive measures included a neuropsychological battery at baseline, 20 weeks, and 40 weeks. MRI scans were obtained pre- and post-intervention. Increases in brain volume were seen in the tai chi and social intervention groups, and the social group also improved on measures of verbal fluency.
The Experience Corps is a multimodal social services program that trains older volunteers to work in schools as tutors and mentors.2 A pilot study of Experience Corps, which randomized participants to placements in elementary schools for 15 hours per week or to a waiting list (control group), demonstrated improvements in executive function and memory in the elementary school volunteers but declines in control group members (Carlson et al., 2009). Older adults at increased risk for cognitive impairment showed intervention-specific short-term gains in executive functioning and in the activity of prefrontal cortical regions, as demonstrated through fMRI (Carlson et al., 2009).
Summary
Although evidence from some epidemiological and observational studies indicates that increases in social activity and social engagement may be associated with higher levels of cognition (Bielak, 2010; Brown et al., 2012; Carlson et al., 2009; Stine-Morrow et al., 2008), evidence from RCTs is needed before recommendations can be made for specific social interaction interventions. Social engagement is often integral to other activities, such as physical activity or participation in the arts, that also have shown cognitive benefits (see Chapter 4C), and fostering social activity in older adults likely has multiple benefits.
DIET
Various dietary patterns have been studied in the hope of identifying nutritional approaches to preserving brain function and preventing cognitive decline. Among these are diets characterized by a higher intake of fruits and vegetables and a lower intake of meat, including the Mediterranean diet and the DASH (Dietary Approaches to Stop Hypertension) diet.
Mediterranean diets, based on the dietary patterns prominent in countries bordering the Mediterranean Sea, emphasize:
- a high consumption of fruits, vegetables, cereals, and legumes;
- a moderate consumption of fish and alcohol, especially red wine; and
- a low consumption of saturated fats (with olive oil as the main source) and dairy products (with yogurt and cheese as the main sources).
The DASH diet emphasizes vegetables, fruits, and fat-free or low-fat dairy products. It includes whole grains, fish, poultry, beans, seeds, nuts, and vegetable oil, and it limits sodium, sweets, sugary beverages, and red meats. In terms of nutrition content, DASH is low in saturated and trans-fats and rich in potassium, calcium, magnesium, fiber, and protein (NIH, 2014a).
Evidence from Observational Studies
A 2010 review cited two studies in which the Mediterranean diet was associated with a decreased risk of cognitive decline (Feart et al., 2009; Plassman et al., 2010; Scarmeas et al., 2009), but it concluded that the quality of the available evidence was low at that time. In two more recent studies, Cache County and Health ABC, global cognitive function scores were found to be significantly higher among those who most adhered to the Mediterranean diet than among those who followed it the least (p = 0.001), and these differences were consistent over an extended follow-up (Koyama et al., 2014; Wengreen et al., 2013). Similar findings were seen for the DASH diet.
By contrast, a study of 6,174 women age 65 years and older who were participating in the Women's Health Study found no associations between the Mediterranean diet and changes in global cognition and verbal memory scores over 5 years (Samieri et al., 2013). In that study, better average global cognition scores were associated with whole-grain intake, and less cognitive decline was seen among those with higher intake of monosaturated versus saturated fats. In Sweden, a 5-year study of 194 older adults without cognitive impairment found that the Mediterranean diet, particularly a low intake of red meat, was associated with better cognitive performance and larger total brain volumes (Titova et al., 2013). Finally, in a study of 2,326 adults in their 70s who were followed for 8 years (38 percent of whom were African American), the Mediterranean diet was associated with slower cognitive decline in African Americans but not in white Caucasians (Koyama et al., 2014). A recent review of 11 observational epidemiologic studies and 1 RCT found that most (9 of 12 studies) indicated that a stricter adherence to the Mediterranean diet was associated with better cognitive function, slower cognitive decline, and a lower risk of developing Alzheimer's disease (Lourida et al., 2013).
People who follow the Mediterranean diet may differ in a number of ways from those who do not, including in such areas as genetic factors, education, SES, physical activity, and other behavioral and cultural characteristics related to cognition. Long-term RCTs that attempt to change people's dietary patterns are difficult and expensive, yet many scientists have called for these in order to more firmly determine whether the Mediterranean diet can actually slow cognitive aging.
The intake of n-3 fatty acids is one aspect of the Mediterranean diet that has received a great deal of attention. These n-3 fatty acids are found in fish that have a high fat content, such as salmon. Fatty acids in the n-3 and n-6 categories are labeled “essential” because the human body does not produce them; food and supplements with these fatty acids are needed to avoid nutritional deficiencies (Beydoun et al., 2014a). Fatty-acid levels in brain cell membranes have been shown to reflect dietary intake (Beydoun et al., 2014a; Haag, 2003), and it has been postulated that n-3 fatty acid intake can increase neurotransmission and the density of neurotransmitter receptors for acetylcholine and dopamine, which in turn can improve learning and memory (Beydoun et al., 2014a; Haag, 2003). A review of five prospective studies concluded that cognitive decline might be reduced by n-3 fatty acid intake but the quality of the evidence was rated low, in part because a single RCT found no such association (Plassman et al., 2010). Seven of 18 prospective studies and five cross-sectional studies found associations between n-3 fatty acid measures and cognitive outcomes (Beydoun et al., 2014a). No cross-sectional or longitudinal associations between the plasma levels of two n-3 fatty acids (docosahexaenoic acid and eicosapentaenoic acid) and seven domains of cognitive function (fine motor speed, verbal memory, visual memory, spatial ability, verbal knowledge, verbal fluency, and working memory) were found in 2,157 U.S. women 65 years old and older who were examined annually over about 6 years of follow-up (Ammann et al., 2013). One study of 390 older adults obtained measures of lifetime fish intake, including both current intakes and reported intake in childhood, and also measured levels of n-3 fatty acid in red blood cell membranes, and all of these measures were correlated with performance on several cognitive speed constructs (Danthiir et al., 2014). However, in 86 older adults who were followed for 4 years (average age = 86 years), higher plasma levels of n-3 fatty acids were associated with less decline in executive function per year of aging, although not with verbal memory or global cognition (Bowman et al., 2013).
Evidence from Intervention Studies
Little intervention evidence supports the benefit of a Mediterranean or DASH diet for cognitive function. However, two secondary analyses of dietary intervention trials for cardiovascular disease outcomes did report positive results. In one, people following the DASH diet combined with a behavioral weight management program exhibited greater improvements in executive function memory learning and in psychomotor speed, and participants following the DASH diet alone exhibited better psychomotor speed than did those in a control group following their usual diet (Smith et al., 2010a). In a Spanish trial comparing two Mediterranean diets (supplemented with either extra-virgin olive oil or mixed nuts) versus a low-fat control diet, those following Mediterranean diets had higher scores on some tests (MMSE and Clock Drawing Test) after 6.5 years of nutritional intervention (Martinez-Lapiscina et al., 2013). However, several potential limitations of these studies must be noted: sample sizes were small, few outcomes were measured, and the populations may not be representative.
Summary
Based on observational studies and limited clinical trials, dietary interventions such as the DASH and Mediterranean diets appear to have promise but their efficacy and effectiveness need to be confirmed in additional clinical trials and community-based studies. While interesting and promising in some studies, the evidence regarding their effects on cognitive aging is too inconclusive to warrant recommendations for dietary change. Yet the evidence summarized here provides some justification for individual choices to eat less meat and more nuts and legumes, whole grains, and monosaturated fats, such as olive oil, to preserve cognitive health. These food choices are consistent with current dietary guidelines for Americans (USDA, 2014).
VITAMINS
Despite widespread publicity about the benefits of vitamins and supplements for brain health and the large expenditures made on these products for a wide variety of reasons (in 2012, $32.5 billion was spent on dietary supplements, including $13.1 billion for vitamin- and mineral-containing supplements [NIH, 2013]), the evidence for supplements enhancing cognition or preventing decline is limited. Much of the published literature is based on cross-sectional or longitudinal observational studies and few RCTs. In addition, methodological differences among the published studies hinder conclusions. These differences include variable doses of the nutrient, different timings of the intervention (e.g., midlife versus late life), different lengths of follow-up, variable adherence (for prospective studies), and variations in recall (for retrospective studies). The evidence base is further complicated by the fact that the same nutrient may have different effects when it is delivered as a supplement from when it is obtained through increased dietary intake. Similarly, foods and beverages may have other components (e.g., polyphenols in coffee) that may be at least partly responsible for an effect attributed to a particular item (e.g., caffeine). Moreover, supplements are not regulated by the Food and Drug Administration and may not be pure and may not contain consistent doses of the active ingredients.
Antioxidants
Antioxidants inhibit the oxidation of other molecules. Oxidation produces free radicals that damage organ systems, including the brain (Christen, 2000; Nunomura et al., 2006). Vitamins C and E, flavonoids, and betacarotene are antioxidants that commonly occur in food and that are also available as supplements. Of particular interest to researchers has been vitamin E—a potent antioxidant that has beneficial effects on a variety of brain structures and functions (e.g., beta-amyloid deposition, loss of neurons, neuroinflammation) associated with cognitive decline in animal and laboratory studies (Nunomura et al., 2006).
Several recent systematic reviews of prospective and cross-sectional studies have found no strong evidence that dietary intakes of antioxidants preserves cognition (Beydoun et al., 2014a; Crichton et al., 2013; Rafnsson et al., 2013). However, in the National Institute on Aging (NIA) systematic review, 9 of 21 prospective studies and 2 of 6 cross-sectional studies did find positive associations between antioxidant intakes, especially vitamin E, and cognitive outcomes (Beydoun et al., 2014a).
A large RCT of vitamin E within the Women's Health Initiative demonstrated no overall benefit, except for women with a low dietary intake of the vitamin (Kang et al., 2006). Moreover, high doses of vitamin E may increase the overall risk of mortality (particularly at doses higher than 400 international units per day, IU/d) (Miller et al., 2005). Finally, a recent systematic review of cross-sectional and longitudinal studies found little or mixed evidence supporting the intake of vitamin E, vitamin C, flavonoids, or carotenoids as a way to improve cognitive function (Crichton et al., 2013).
B Vitamins and Homocysteine
Elevated homocysteine, a sulfur amino acid in the blood, has been consistently associated with increased risk of heart disease and stroke, although it is not known to what extent these associations are explained by poor kidney function which allows homocysteine to build up in the blood (He et al., 2014; Humphrey et al., 2008). Homocysteine levels increase with age (as do markers of kidney function aging) but intervention trials show that homocysteine levels in the blood can be lowered by supplemental intake of B6 and B12 vitamins and folate (Clarke et al., 2014).
Recently, Canadian researchers conducted an evidence-based review to determine the answers to several important questions about homocysteine and vitamin B12 in relation to cognitive decline and dementia (OHTAC, 2013). What seems most clear from this effort is that homocysteine is consistently, though not universally, associated with poor cognitive outcomes. In the NIA systematic review, 12 of 19 prospective studies and 11 of 14 prospective studies found higher homocysteine levels to be associated with poor cognitive outcomes (Beydoun et al., 2014a). However, associations between B vitamin intake and blood levels of B vitamins and cognitive health are inconclusive, with the Canadian report concluding that B12 supplementation did not appreciably change cognitive function or decline based on moderately strong evidence (OHTAC, 2013).
In a small clinical trial among people with mild B12 deficiency, supplementation did not improve cognition (Eussen et al., 2006).
Folate
Folate supplementation has not produced improved cognitive benefit among people in general (Malouf and Grimley Evans, 2008; Wald et al., 2010), although some benefit has been shown among those who have folic acid insufficiency as indicated by high homocysteine levels at baseline (Durga et al., 2007).
Vitamin D
Several prospective epidemiologic studies have shown associations of low serum vitamin D levels with lower global cognition and a more rapid functional decline (Annweiler and Beauchet, 2014; Bartali et al., 2014; Slinin et al., 2012; Wilson et al., 2014), although this association may not be relevant for African Americans, who in general have lower serum vitamin D levels than white Caucasians (Schneider et al., 2014). In a recent review of 10 epidemiologic studies, the serum level for vitamin D most clearly demarcating insufficiency for cognitive health was found to be around 10 nanograms/milliliter (Annweiler and Beauchet, 2014). It is less clear whether and to what extent vitamin D supplements would improve cognition or prevent its decline in older populations. Moreover, high levels of serum 25-hydroxyvitamin D, especially among those taking vitamin D supplements, are associated with cognitive impairment on a battery of attention tests, suggesting that the benefit may be confined to middle-range serum levels (Granic et al., 2014).
In the Women's Health Initiative Memory Study, women randomized to receive 1,000 mg of calcium carbonate and 400 IU/d of vitamin D3 did not show differences in performance (attention and working memory, working memory, word knowledge, spatial ability, verbal fluency, verbal memory, figural memory, or fine motor speed) over 7.8 years (Rossom et al., 2012). An RCT of vitamin D (5,000 IU/d of cholecalciferol) in young adults demonstrated no significant changes compared to placebo on measures related to working memory, response inhibition, or cognitive flexibility (Dean et al., 2011). Hopefully, future large RCTs testing vitamin D supplements will shed light on this question (Rossom et al., 2012).
Summary
The medical literature does not convincingly support any vitamin supplement intervention to prevent cognitive decline. There is evidence to support the replacement of folate among older people who are folate-deficient, as evidenced by high homocysteine levels, but not for the supplementation of older persons who are not deficient. Whether it is worthwhile to screen for these deficiencies in general populations remains an unanswered question. To date, the U.S. Preventive Services Task Force has not issued recommendations regarding such screenings.
ALCOHOL
Excessive alcohol consumption has detrimental effects on judgment and reaction times and can cause long-term cognitive damage (Bartley and Rezvani, 2012). The reasons for this include altered nutrition; the consequences of excessive alcohol consumption, such as cirrhosis; interactions with medications and other substances; and alcohol-associated injuries, such as those sustained in vehicular crashes. Furthermore, high levels of alcohol intake may be associated with psychiatric comorbidity, which also may have adverse cognitive consequences. Compared to either abstinence or excessive alcohol consumption, moderate drinking in adulthood—generally less than two drinks per day and in most populations much less than this—is associated with various health benefits such as the avoidance of heart disease and a lower mortality risk (Jayasekara et al., 2014; Roerecke and Rehm, 2012), although consideration needs to be given to baseline cognitive function (Krahn et al., 2003). This J- or U-shaped relationship between alcohol consumption and some health outcomes has been postulated to extend to cognitive aging and dementia. Possible mechanisms for the lower rates of cognitive and other impairments associated with moderate alcohol intake include decreased cardiovascular risk, improved lipid levels, the antioxidant properties of flavonoids found in red wine, lower platelet aggregation, improved insulin sensitivity, reduced inflammation, and possibly a direct effect of alcohol on cognitive function through acetylcholine release in the hippocampus that may improve learning and memory (Beydoun et al., 2014a; Peters et al., 2008a). Moderate, controlled intake may also be a behavioral indicator of personal control regarding risky behaviors. A 2008 systematic review examined 23 studies involving adults age 65 years and older (20 prospective and 3 case-control studies nested in a cohort) and found a nonsignificant pooled relative risk for cognitive decline (RR = 0.89; 0.67–1.17) (Peters et al., 2008a). In the NIA systematic review, 8 of 18 prospective studies found the J- or U-shaped association between moderate alcohol consumption and cognitive outcomes, as did 9 of 12 cross-sectional studies (Beydoun et al., 2014a).
Observational evidence suggests that light to moderate alcohol consumption is not a risk factor for the loss of cognitive function and may even be a protective factor throughout adulthood. However, the evidence base is insufficient to recommend an amount of alcohol intake, if any, that would be beneficial for cognition, particularly across the life span. Excessive alcohol consumption has well-established cognitive harms (Peters et al., 2008a).
SMOKING
Smoking is a strong risk factor for heart disease, stroke, cancer, lung disease, and many other chronic conditions (CDC, 2008). Its relationship to cognitive outcomes is complex. Given that smoking increases the risk of stroke, it would be expected also to increase the risk of vascular dementia and cognitive decline. However, the nicotine in cigarettes increases the release of acetylcholine, which improves attention and information processing (Beydoun et al., 2014a). Moreover, deficits in the cholinergic system are involved in Alzheimer's disease, and thus it has been suggested that smoking can delay Alzheimer's disease (Beydoun et al., 2014a). The death rate of smokers is three times that of non-smokers, and their life expectancy is shortened by approximately 10 years (Jha et al., 2013). Thus, it may be that the lower incidence of Alzheimer's disease among smokers is an artifact of their earlier deaths from other causes. Furthermore, systematic reviews have found that current smokers have an increased age-specific risk of Alzheimer's disease, vascular dementia, and other dementias (Peters et al., 2008b), while these risks were not significantly increased among former smokers.
In the NIA systematic review, 16 of 29 prospective studies observed associations between smoking and poorer cognitive outcomes, while four studies found associations with some outcomes or in some subgroups, and nine studies found no associations or associations in which smokers had better cognitive outcomes than non-smokers (Beydoun et al., 2014a). Only two of seven cross-sectional studies found associations of smoking with poorer cognitive outcomes. Several large studies have found associations with cognitive decline, dementia, and Alzheimer's disease in heavy smokers, but not in lighter smokers (Beydoun et al., 2014a).
To the committee's knowledge, no intervention trials have been conducted that manipulate smoking initiation or smoking cessation and then examine the effects on cognitive outcomes.
The evidence linking smoking to cognitive outcomes is mixed and inconclusive. Given the many health problems of aging smokers and the relationship of smoking to coronary disease, stroke, and dementias, there are ample reasons for current smokers to quit, despite the uncertainty about the cognitive benefits.
SUBSTANCE ABUSE
Evidence from Observational Studies
Several substances commonly abused in Western societies have been suggested as contributing to cognitive impairment, including cannabis, particularly in teenagers and young adults (Becker et al., 2014; Jacobus and Tapert, 2014; Thames et al., 2014). Much of this impairment comes during the acute and post-acute phases of use (Crane et al., 2013), and evidence suggests that among adolescents who stop using cannabis, there are no residual cognitive effects (Hooper et al., 2014). Also, little evidence suggests that cannabis use has an adverse impact on cognitive performance among older adults (van Holst and Schilt, 2011).
Methamphetamine is a highly addictive and neurotoxic psychostimulant that in experimental animals produces long-lasting memory deficits (North et al., 2013). However, in humans, the long-term effects of chronic methamphetamine use on cognition are less clear. In a review of this drug as a cause of cognitive decline in humans, the findings were judged to be mixed (Dean et al., 2013), and some cognitive decrements could be attributed to other factors or to variation in study designs. In one large study of chronic methamphetamine users, lifetime use was not related to cognitive functioning per se, but it was related to psychiatric comorbidity and the use of other substances, such as crack cocaine (Herbeck and Brecht, 2013).
Chronic opiate use has been associated with poorer cognitive performance in human studies (Darke et al., 2012; Terrett et al., 2014; van Holst and Schilt, 2011), but, as above, several problems arise in interpreting this research, including the presence of psychiatric comorbidity and neurotoxic exposures. A systematic review of the impact of methadone, an opioid congener used in the treatment of opiate addiction, suggested that impaired cognitive function was correlated with its use (Wang et al., 2013).
While all of these substances have proved neurotoxic, producing adverse neurodevelopmental effects in both animal models and humans, there is a dearth of information on long-term cognitive performance in older adults who were former substance abusers. Hospitalizations for substance abuse among older persons have recently increased (Wu and Blazer, 2011), but available data—limited primarily to hospital claims data—so far do not suggest increased cognitive comorbidity as a result (Wu et al., 2013).
Further research is needed to better understand the long-term cognitive consequences of substance abuse and the changes in cognitive function that are associated with the treatment of drug addictions. If long-term adverse effects emerge, they would provide added impetus for drug abuse prevention at all ages and possible special preventive and therapeutic approaches for those with drug abuse histories.
PHYSICAL ENVIRONMENT
Older people experience a number of general as well as occupational exposures that may adversely affect cognitive performance. The role of potentially adverse exposures that occur early in life on cognitive trajectories is often uncertain, in part because of the challenges of performing the critical research needed, but also because of the multiple confounding exposures and experiences that occur across a lifetime. However, middle-aged and older individuals may also have continuing environmental exposures that may alter their cognitive performance. Some of the more common and potentially important exposures are described here.
Air Pollution
Growing evidence indicates that general, ambient air pollution is associated with decrements in cognitive performance among middle-aged and older adults (Ailshire and Clarke, 2014; Ailshire and Crimmins, 2014; Fonken et al., 2011; Gatto et al., 2014). However, because most air pollution is a complex mixture of gaseous and particulate components, it is difficult to attribute the effect to a specific factor. Most epidemiological studies assess the level of particulate matter less than 2.5 microns in diameter as the index of exposure (Ailshire and Crimmins, 2014). Higher levels of air pollution are sometimes accompanied by adverse climatic conditions, such as extremely high temperatures, which also can have adverse health effects (Gold and Mittleman, 2013). Air pollutants may have other important, if indirect, effects on cognitive function through their contributions to the development of cardiopulmonary conditions, including ischemic heart disease, stroke, lung conditions (Gold and Mittleman, 2013; Shah et al., 2013), and other chronic illnesses (see Chapter 4B). Taken together, such effects provide an argument for the environmental control of air pollution in general and for the prevention of exposures among older adults.
Occupational Exposures
For many years, occupational environmental exposures of various types have been suspected of having adverse cognitive consequences. This is a large and complex literature on this subject, and only certain exposures will be discussed here. As in the assessment of other risk factors, cognitive decrements associated with occupational exposures have been easier to demonstrate with concurrent exposure—and less research having been done on the long-term consequences, especially as they may affect older adults (Genuis and Kelln, 2015).
One important set of work-related chemical exposures that has been the subject of substantial research is exposure to organophosphate pesticides. Most but not all reviews of this topic suggest that job-related exposures do lead to cognitive decrements in late life (Blanc-Lapierre et al., 2013). Animal models also suggest that continued later-life exposure may be harmful (Levin et al., 2010), but no important human evidence is available.
The neurotoxic effects of lead exposure have been known for centuries, and the effect of lead on children has been a prominent public health issue. Studies of occupational lead exposure find that decrements in cognitive performance caused by earlier job-related exposure extend into late mid-life and that among older adults higher levels of lead in the bone are correlated with worse cognitive performance (Khalil et al., 2009). Lead exposure is one of those environmental and occupational exposures that will require ongoing attention and preventive interventions in the future.
Another category of occupational exposures that has received research attention is exposure to organic solvents. While these substances' acute and subacute toxic effects have been demonstrated, their late-life cognitive decrements due to chronic exposures have not been firmly established, although there is some evidence suggesting that there are long-term cognitive changes (Berr et al., 2010). For example, long-lasting neuropsychiatric symptoms associated with heavy solvent exposure have been found among tile layers (Nordling Nilson et al., 2007), although a subsequent analysis of the data from Berr and colleagues (2010) found that the cognitive effects appeared only among individuals with lesser educational attainment (Sabbath et al., 2012), suggesting that not all individuals are equally at risk from a given exposure.
While knowledge about the effects of occupational exposures on cognitive performance is an extremely important area for prevention, research on these effects is always challenging: many similar chemical and other exposures occur simultaneously; industrial processes and consequent exposures change with advances in production; exposed workers may be subject to other factors, occupational or non-occupational, that could lead to cognitive decrements; some studies use clinical dementia or specific neurodegenerative diseases as outcomes rather than cognitive aging; and workers in jobs with substantial occupational chemical exposures may have lower levels of education, itself an important risk factor for cognitive change in later life. Despite the uncertainties, one clear message is that whenever workers may be exposed to a substance for which there is reasonable evidence of neurotoxicity, effective exposure prevention programs should be enforced in the workplace at all times to protect both younger and older workers. Furthermore, it would be prudent for any comprehensive cognitive health program to screen older adults for non-occupational exposures to these same neurotoxic chemicals, such as exposures through hobby or recreational activities.
Other potential workplace exposures—such as might occur in workplaces relying heavily on computers and information and communications technologies—can lead to stress, mental health symptoms, and, at least potentially, alterations in cognitive function (Salanova et al., 2013; Thomee et al., 2010). However, it is also possible that the technological features of a job can reduce job stress and demand (Day et al., 2012). Whether technology-related job stress leads to cognitive functional change remains uncertain.
Noise can also be a factor in occupational settings. Environmental noise from traumatic and non-traumatic sources indisputably causes hearing impairment (Gourevitch et al., 2014). Hearing impairment from various causes, including age-related hearing loss, has been associated with reduced cognitive performance (see Chapter 4B). The cognitive performance of older adults experiencing higher levels of ambient noise may be affected indirectly, if it is distracting or reduces concentration (Jahncke, 2012) or interrupts normal sleep patterns (Muzet, 2007). The committee is not aware of any intervention trials that have examined cognitive outcomes after manipulating noise levels.
A more general question is whether occupations, when they are challenging, have a protective effect and lead to more cognitive engagement. While a number of observational studies of this question have been conducted, the results are decidedly mixed, and many of the studies do not show any clear effect of type of occupation on brain function (Finkel et al., 2009; Gow et al., 2014; Jorm et al., 1998).
STRESS
With the publication of Hans Selye's letter to the journal Nature in 1936, the concept of “stress” became a subject of interest to researchers (Szabo et al., 2012). Early on, stress was defined non-specifically as the response of the body to a noxious stimulus, which later became known as “general adaptation syndrome” (Szabo et al., 2012). As the American Institute of Stress notes, “Stress is not a useful term for scientists because it is such a highly subjective phenomenon that it defies definition. And if you can't define stress, how can you possibly measure it?” (AIS, 2014). Thus, it is not surprising that the evidence relating stress to cognitive outcomes has been compiled in diverse ways and has produced results that are inconsistent and difficult to interpret. The “distress” encountered daily comes in many forms—from traffic congestion to the experience of having a bad boss to the death of a loved one—and it raises the question, “Does stress cause cognitive decline?” For this reason, the committee briefly addresses stress here, even though the current evidence base raises more questions than it answers.
Selye's original experiments were done with mice, and indeed a number of studies have assessed the effects of experimental stressors on cognition in laboratory settings. For example, it is known that in both monkeys and rodents, stress affects the pyramidal neurons in the prefrontal cortex, the same neurons that are affected by aging. The interactive effects of stress and aging in the rodent model were recently reviewed in detail (McEwen and Morrison, 2013).
In epidemiological studies, stress is frequently defined in terms of stressful life events (poor parenting in early life, divorce, loss of a job, or death of a loved one), and perceived stress is measured by various validated scales, or personality traits that make some people more vulnerable to stress than others. Finnish boys separated from their parents during World War II were found to have lower cognitive scores in several domains at age 60, with longer separations linked to greater deficits (Pesonen et al., 2007). The death of a child is a major life stressor associated with a faster rate of cognitive decline in later life (Comijs et al., 2011; Greene et al., 2014), especially when the loss occurred among parents who were young adults or had no subsequent children (Greene et al., 2014). However, the number and subjective ratings of stressful life events in the Cache County Study were associated only inconsistently with cognitive decline; stronger associations between stressful life events and cognitive decline were found in younger participants and those with less education (Tschanz et al., 2013). In the Longitudinal Aging Study Amsterdam, the death of a child or grandchild was associated with faster cognitive decline, and having experienced fewer major life events was associated with better cognitive function (Comijs et al., 2011).
Similarly, perceived daily stress has also been associated with greater memory problems among adults with cognitive decline (Rickenbach et al., 2014). Greater levels of perceived stress were also associated with lower initial cognitive performance and faster rates of decline among 6,207 older adults followed for almost 7 years in the Chicago Health and Aging Project (Aggarwal et al., 2014). Perceived social strain has been shown to elevate levels of cortisol (Friedman et al., 2012), a stress hormone and a marker of activation of the hypothalamic–pituitary–adrenal axis. Chronic activation of this axis has been associated with poor health outcomes, including cardiovascular disease, which could also further contribute to cognitive decline and dementia (Kremen et al., 2012).
Few intervention trials have been carried out with the goal of preserving cognition by modifying perceived stress or attempting to reduce stress responses during or after major life events. In a recent review of 12 studies of meditation and mindfulness and cognition in older people, 6 of which were randomized trials, meditation and mindfulness seemed to have benefits for a number of cognitive domains, but the trials were small and susceptible to bias (Gard et al., 2014). Major life events, as well as perceived stress and mild worry, have been associated with decreased cognitive performance and faster cognitive decline in several recent studies. Interventions that reduce the perceptions of stress or responses to it, such as meditation and mindfulness, may be helpful but require further study.
REFERENCES
- Abourezk T, Toole T. Effect of task complexity on the relationship between physical fitness and reaction time in older women. Journal of Aging and Physical Activity. 1995;(3):251–260.
- ACE (American Council on Education) and MetLife Foundation. Framing new terrain: Older adults and higher education. 2007. [on February 26, 2015]. http://plus50
.aacc.nche .edu/documents/older _adults_and_higher_education.pdf. - Aggarwal NT, Wilson RS, Beck TL, Rajan KB, Mendes de Leon CF, Evans DA, Everson-Rose SA. Perceived stress and change in cognitive function among adults 65 years and older. Psychosomatic Medicine. 2014;76(1):80–85. [PMC free article: PMC4185366] [PubMed: 24367123]
- Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clinic Proceedings. 2011;86(9):876–884. [PMC free article: PMC3258000] [PubMed: 21878600]
- Ailshire JA, Clarke P. Fine particulate matter air pollution and cognitive function among U.S. older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2014;70(2):322–328. [PMC free article: PMC4351385] [PubMed: 24906394]
- Ailshire JA, Crimmins EM. Fine particulate matter air pollution and cognitive function among older U.S. adults. American Journal of Epidemiology. 2014;180(4):359–366. [PMC free article: PMC4128773] [PubMed: 24966214]
- AIS (American Institute of Stress). What is stress? 2014. [December 5, 2014]. http://www
.stress.org/what-is-stress. - Albert SM, Teresi JA. Reading ability, education, and cognitive status assessment among older adults in Harlem, New York City. American Journal of Public Health. 1999;89(1):95–97. [PMC free article: PMC1508491] [PubMed: 9987476]
- Ammann EM, Pottala JV, Harris WS, Espeland MA, Wallace R, Denburg NL, Carnahan RM, Robinson JG. Omega-3 fatty acids and domain-specific cognitive aging: Secondary analyses of data from WHISCA. Neurology. 2013;81(17):1484–1491. [PMC free article: PMC3888166] [PubMed: 24068783]
- Anderson D, Seib C, Rasumssen L. Can physical activity prevent physical and cognitive decline in postmenopausal women? A systematic review of the literature. Maturitas. 2014;79(1):14–33. [PubMed: 25008420]
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. The Cochrane Database of Systematic Reviews. 2008;(2):Cd005381. [PubMed: 18425918]
- Annweiler C, Beauchet O. Vitamin D in older adults: The need to specify standard values with respect to cognition. Frontiers in Aging Neuroscience. 2014;6:72. [PMC free article: PMC3995037] [PubMed: 24782767]
- Arenaza-Urquijo EM, Molinuevo JL, Sala-Llonch R, Sole-Padulles C, Balasa M, Bosch B, Olives J, Antonell A, Llado A, Sanchez-Valle R, Rami L, Bartres-Faz D. Cognitive reserve proxies relate to gray matter loss in cognitively healthy elderly with abnormal cerebrospinal fluid amyloid-beta levels. Journal of Alzheimer's Disease. 2013;35(4):715–726. [PubMed: 23481686]
- Barnes DE, Santos-Modesitt W, Poelke G, Kramer AF, Castro C, Middleton LE, Yaffe K. The mental activity and exercise (MAX) trial: A randomized controlled trial to enhance cognitive function in older adults. JAMA Internal Medicine. 2013;173(9):797–804. [PMC free article: PMC5921904] [PubMed: 23545598]
- Bartali B, Devore E, Grodstein F, Kang JH. Plasma vitamin d levels and cognitive function in aging women: The Nurses' Health Study. Journal of Nutrition, Health, and Aging. 2014;18(4):400–406. [PMC free article: PMC4198067] [PubMed: 24676321]
- Bartley PC, Rezvani AH. Alcohol and cognition–Consideration of age of initiation, usage patterns and gender: A brief review. Current Drug Abuse Reviews. 2012;5(2):87–97. [PubMed: 22455507]
- Becker MP, Collins PF, Luciana M. Neurocognition in college-aged daily marijuana users. Journal of Clinical and Experimental Neuropsychology. 2014;36(4):379–398. [PMC free article: PMC4074777] [PubMed: 24620756]
- Berr C, Vercambre MN, Bonenfant S, Manoux AS, Zins M, Goldberg M. Occupational exposure to solvents and cognitive performance in the GAZEL cohort: Preliminary results. Dementia and Geriatric Cognitive Disorders. 2010;30(1):12–19. [PMC free article: PMC2945272] [PubMed: 20606440]
- Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health. 2014a;14:643. [PMC free article: PMC4099157] [PubMed: 24962204]
- Beydoun MA, Gamaldo AA, Beydoun HA, Tanaka T, Tucker KL, Talegawkar SA, Ferrucci L, Zonderman AB. Caffeine and alcohol intakes and overall nutrient adequacy are associated with longitudinal cognitive performance among U.S. adults. Journal of Nutrition. 2014b;144(6):890–901. [PMC free article: PMC4018952] [PubMed: 24744319]
- Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. Journal of Aging Research. 2013 657508. [PMC free article: PMC3786463] [PubMed: 24102028]
- Bielak AA. How can we not “lose it” if we still don't understand how to “use it”? Unanswered questions about the influence of activity participation on cognitive performance in older age. A mini-review. Gerontology. 2010;56(5):507–519. [PubMed: 19996570]
- Blanc-Lapierre A, Bouvier G, Gruber A, Leffondre K, Lebailly P, Fabrigoule C, Baldi I. Cognitive disorders and occupational exposure to organophosphates: Results from the PHYTONER study. American Journal of Epidemiology. 2013;177(10):1086–1096. [PubMed: 23535900]
- Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014;14(510) [PMC free article: PMC4064273] [PubMed: 24885250]
- Blumenthal JA, Emery CF, Madden DJ, Schniebolk S, Walsh-Riddle M, George LK, McKee DC, Higginbotham MB, Cobb FR, Coleman RE. Long-term effects of exercise on psychological functioning in older men and women. Journal of Gerontology. 1991;46(6):P352–P361. [PubMed: 1940092]
- Bowman GL, Dodge HH, Mattek N, Barbey AK, Silbert LC, Shinto L, Howieson DB, Kaye JA, Quinn JF. Plasma omega-3 PUFA and white matter mediated executive decline in older adults. Frontiers in Aging Neuroscience. 2013;5:92. [PMC free article: PMC3863786] [PubMed: 24379780]
- Brown CL, Gibbons LE, Kennison RF, Robitaille A, Lindwall M, Mitchell MB, Shirk SD, Atri A, Cimino CR, Benitez A, Macdonald SW, Zelinski EM, Willis SL, Schaie KW, Johansson B, Dixon RA, Mungas DM, Hofer SM, Piccinin AM. Social activity and cognitive functioning over time: A coordinated analysis of four longitudinal studies. Journal of Aging Research. 2012 287438. [PMC free article: PMC3444000] [PubMed: 22991665]
- Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, Hayasaka S, Jennings JM, Katula JA, Kraft RA, Rejeski WJ. Using network science to evaluate exercise-associated brain changes in older adults. Frontiers in Aging Neuroscience. 2010;2(23) [PMC free article: PMC2893375] [PubMed: 20589103]
- Burn KF, Henderson VW, Ames D, Dennerstein L, Szoeke C. Role of grandparenting in postmenopausal women's cognitive health: Results from the Women's Healthy Aging Project. Menopause. 2014;21(10):1069–1074. [PubMed: 24714623]
- Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends in Cognitive Sciences. 2009;13(10):447–454. [PMC free article: PMC2752489] [PubMed: 19726219]
- Carlson MC, Erickson KI, Kramer AF, Voss MW, Bolea N, Mielke M, McGill S, Rebok GW, Seeman T, Fried LP. Evidence for neurocognitive plasticity in at-risk older adults: The Experience Corps program. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2009;64(12):1275–1282. [PMC free article: PMC2781785] [PubMed: 19692672]
- CDC (Centers for Disease Control and Prevention). Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. Morbidity and Mortality Report. 2008;57(45):1226–1228. [PubMed: 19008791]
- Christen Y. Oxidative stress and Alzheimer disease. American Journal of Clinical Nutrition. 2000;71(2):621s–629s. [PubMed: 10681270]
- Clarke R, Bennett D, Parish S, Lewington S, Skeaff M, Eussen SJ, Lewerin C, Stott DJ, Armitage J, Hankey GJ, Lonn E, Spence JD, Galan P, de Groot LC, Halsey J, Dangour AD, Collins R, Grodstein F. Effects of homocysteine lowering with B vitamins on cognitive aging: Meta-analysis of 11 trials with cognitive data on 22,000 individuals. American Journal of Clinical Nutrition. 2014;100(2):657–666. [PMC free article: PMC4095663] [PubMed: 24965307]
- Clarkson-Smith L, Hartley AA. Structural equation models of relationships between exercise and cognitive abilities. Psychology and Aging. 1990;5(3):437–446. [PubMed: 2242248]
- Cohn NB, Dustman RE, Bradford DC. Age-related decrements in Stroop color test performance. Journal of Clinical Psychology. 1984;40(5):1244–1250. [PubMed: 6490922]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–130. [PubMed: 12661673]
- Colcombe SJ, Kramer AF, McAuley E, Erickson KI, Scalf P. Neurocognitive aging and cardiovascular fitness: Recent findings and future directions. Journal of Molecular Neuroscience. 2004;24(1):9–14. [PubMed: 15314244]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2006;61(11):1166–1170. [PubMed: 17167157]
- Comijs HC, van den Kommer TN, Minnaar RW, Penninx BW, Deeg DJ. Accumulated and differential effects of life events on cognitive decline in older persons: Depending on depression, baseline cognition, or ApoE epsilon4 status? Journals of Gerontology: Series B, Psychological Sciences and Social Sciences. 2011;66(Suppl 1):i111–i120. [PubMed: 21422053]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25(6):295–301. [PubMed: 12086747]
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R. Effects of cannabis on neurocognitive functioning: Recent advances, neurodevelopmental influences, and sex differences. Neuropsychology Review. 2013;23(2):117–137. [PMC free article: PMC3593817] [PubMed: 23129391]
- Crichton GE, Bryan J, Murphy KJ. Dietary antioxidants, cognitive function and dementia—A systematic review. Plant Foods for Human Nutrition. 2013;68(3):279–292. [PubMed: 23881465]
- Danthiir V, Hosking D, Burns NR, Wilson C, Nettelbeck T, Calvaresi E, Clifton P, Wittert GA. Cognitive performance in older adults is inversely associated with fish consumption but not erythrocyte membrane n-3 fatty acids. Journal of Nutrition. 2014;144(3):311–320. [PubMed: 24353345]
- Darke S, McDonald S, Kaye S, Torok M. Comparative patterns of cognitive performance amongst opioid maintenance patients, abstinent opioid users and non-opioid users. Drug and Alcohol Dependence. 2012;126(3):309–315. [PubMed: 22726911]
- Day A, Paquet S, Scott N, Hambley L. Perceived information and communication technology (ICT) demands on employee outcomes: The moderating effect of organizational ICT support. Journal of Occupational Health Psychology. 2012;17(4):473–491. [PubMed: 23066697]
- Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2013;38(2):259–274. [PMC free article: PMC3527116] [PubMed: 22948978]
- Dean AJ, Bellgrove MA, Hall T, Phan WM, Eyles DW, Kvaskoff D, McGrath JJ. Effects of vitamin D supplementation on cognitive and emotional functioning in young adults—a randomised control trial. PLoS ONE. 2011;6(11):e25966. [PMC free article: PMC3208539] [PubMed: 22073146]
- Deary IJ. Intelligence. Annual Review of Psychology. 2012;63:453–482. [PubMed: 21943169]
- Durga J, van Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, Verhoef P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet. 2007;369(9557):208–216. [PubMed: 17240287]
- Dustman RE, Ruhling RO, Russell EM, Shearer DE, Bonekat HW, Shigeoka JW, Wood JS, Bradford DC. Aerobic exercise training and improved neuropsychological function of older individuals. Neurobiology of Aging. 1984;5(1):35–42. [PubMed: 6738784]
- EClipSE Collaborative Members. Brayne C, Ince PG, Keage HA, McKeith IG, Matthews FE, Polvikoski T, Sulkava R. Education, the brain and dementia: Neuroprotection or compensation? Brain. 2010;133(Pt 8):2210–2216. [PubMed: 20826429]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, Kuller LH. Physical activity predicts gray matter volume in late adulthood: The Cardiovascular Health Study. Neurology. 2010;75(16):1415–1422. [PMC free article: PMC3039208] [PubMed: 20944075]
- Eussen SJ, de Groot LC, Joosten LW, Bloo RJ, Clarke R, Ueland PM, Schneede J, Blom HJ, Hoefnagels WH, van Staveren WA. Effect of oral vitamin B-12 with or without folic acid on cognitive function in older people with mild vitamin B-12 deficiency: A randomized, placebo-controlled trial. American Journal of Clinical Nutrition. 2006;84(2):361–370. [PubMed: 16895884]
- Feart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, Barberger-Gateau P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302(6):638–648. [PMC free article: PMC2850376] [PubMed: 19671905]
- Federman AD, Sano M, Wolf MS, Siu AL, Halm EA. Health literacy and cognitive performance in older adults. Journal of the American Geriatrics Society. 2009;57(8):1475–1480. [PMC free article: PMC2754116] [PubMed: 19515101]
- Finkel D, Andel R, Gatz M, Pedersen NL. The role of occupational complexity in trajectories of cognitive aging before and after retirement. Psychology and Aging. 2009;24(3):563–573. [PMC free article: PMC2742987] [PubMed: 19739912]
- Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S, Nelson RJ. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Molecular Psychiatry. 2011;16(10):987–995. [PMC free article: PMC3270364] [PubMed: 21727897]
- Friedman EM, Karlamangla AS, Almeida DM, Seeman TE. Social strain and cortisol regulation in midlife in the U.S. Social Science and Medicine. 2012;74(4):607–615. [PMC free article: PMC3605735] [PubMed: 22209675]
- Gard T, Hölzel BK, Lazar SW. The potential effects of meditation on age-related cognitive decline: A systematic review. Annals of the New York Academy of Sciences. 2014;1307(1):89–103. [PMC free article: PMC4024457] [PubMed: 24571182]
- Gatto NM, Henderson VW, Hodis HN, St John JA, Lurmann F, Chen JC, Mack WJ. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology. 2014;40:1–7. [PMC free article: PMC3946571] [PubMed: 24148924]
- Geda YE, Topazian HM, Roberts LA, Roberts RO, Knopman DS, Pankratz VS, Christianson TJ, Boeve BF, Tangalos EG, Ivnik RJ, Petersen RC. Engaging in cognitive activities, aging, and mild cognitive impairment: A population-based study. Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23(2):149–154. [PMC free article: PMC3204924] [PubMed: 21677242]
- Genuis SJ, Kelln KL. Toxicant exposure and bioaccumulation: A common and potentially reversible cause of cognitive dysfunction and dementia. Behavioural Neurology. 2015 620143. [PMC free article: PMC4334623] [PubMed: 25722540]
- Gold DR, Mittleman MA. New insights into pollution and the cardiovascular system: 2010 to 2012. Circulation. 2013;127(18):1903–1913. [PubMed: 23648681]
- Gourevitch B, Edeline JM, Occelli F, Eggermont JJ. Is the din really harmless? Long-term effects of non-traumatic noise on the adult auditory system. Nature Reviews Neuroscience. 2014;15(7):483–491. [PubMed: 24946762]
- Gow AJ, Bielak AA, Gerstorf D. Lifestyle factors and cognitive ageing: Variation across ability and lifestyle domains. Journal of Aging Research. 2012 143595. [PMC free article: PMC3502015] [PubMed: 23193475]
- Gow AJ, Avlund K, Mortensen EL. Occupational characteristics and cognitive aging in the Glostrup 1914 Cohort. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2014;69(2):228–236. [PubMed: 23223088]
- Granic A, Hill TR, Kirkwood TB, Davies K, Collerton J, Martin-Ruiz C, von Zglinicki T, Saxby BK, Wesnes KA, Collerton D, Mathers JC, Jagger C. Serum 25-hydroxyvitamin D and cognitive decline in the very old: The Newcastle 85+ Study. European Journal of Neurology. 2014;22(1):106–115. [PMC free article: PMC4310141] [PubMed: 25117780]
- Greene D, Tschanz JT, Smith KR, Ostbye T, Corcoran C, Welsh-Bohmer KA, Norton MC. Impact of offspring death on cognitive health in late life: The Cache County study. American Journal of Geriatric Psychiatry. 2014;22(11):1307–1315. [PMC free article: PMC3923854] [PubMed: 23954042]
- Haag M. Essential fatty acids and the brain. Canadian Journal of Psychiatry. 2003;48(3):195–203. [PubMed: 12728744]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise. 2007;39(8):1423–1434. [PubMed: 17762377]
- He Y, Li Y, Chen Y, Feng L, Nie Z. Homocysteine level and risk of different stroke types: A meta-analysis of prospective observational studies. Nutrition, Metabolism, and Cardiovascular Diseases. 2014;24(11):1158–1165. [PubMed: 24984821]
- Hendrie HC, Albert MS, Butters MA, Gao S, Knopman DS, Launer LJ, Yaffe K, Cuthbert BN, Edwards E, Wagster MV. The NIH Cognitive and Emotional Health Project. Report of the Critical Evaluation Study Committee. Alzheimer's & Dementia. 2006;2(1):12–32. [PubMed: 19595852]
- Herbeck DM, Brecht ML. Substance use and mental health characteristics associated with cognitive functioning among adults who use methamphetamine. Journal of Addictive Diseases. 2013;32(1):11–25. [PMC free article: PMC3601587] [PubMed: 23480244]
- HHS (U.S. Department of Health and Human Services). Healthy people. 2014a. [October 27, 2014]. http://www
.healthypeople.gov. - HHS. Healthy people 2020 leading health indicators: Nutrition, physical activity, and obesity. 2014b. [January 21, 2015]. http://www
.healthypeople .gov/sites/default /files/HP2020_LHI_Nut_PhysActiv.pdf. - Holwerda TJ, Deeg DJ, Beekman AT, van Tilburg TG, Stek ML, Jonker C, Schoevers RA. Feelings of loneliness, but not social isolation, predict dementia onset: Results from the Amsterdam Study of the Elderly (AMSTEL). Journal of Neurology, Neurosurgery, and Psychiatry. 2014;85(2):135–142. [PubMed: 23232034]
- Hooper SR, Woolley D, De Bellis MD. Intellectual, neurocognitive, and academic achievement in abstinent adolescents with cannabis use disorder. Psychopharmacology. 2014;231(8):1467–1477. [PMC free article: PMC3969383] [PubMed: 24619597]
- Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: A systematic review and meta-analysis. Mayo Clinic Proceedings. 2008;83(11):1203–1212. [PubMed: 18990318]
- Jacobus J, Tapert SF. Effects of cannabis on the adolescent brain. Current Pharmaceutical Design. 2014;20(13):2186–2193. [PMC free article: PMC3930618] [PubMed: 23829363]
- Jahncke H. Open-plan office noise: The susceptibility and suitability of different cognitive tasks for work in the presence of irrelevant speech. Noise and Health. 2012;14(61):315–320. [PubMed: 23257585]
- Jayasekara H, English DR, Room R, MacInnis RJ. Alcohol consumption over time and risk of death: A systematic review and meta-analysis. American Journal of Epidemiology. 2014;179(9):1049–1059. [PubMed: 24670372]
- Jefferson AL, Gibbons LE, Rentz DM, Carvalho JO, Manly J, Bennett DA, Jones RN. A life course model of cognitive activities, socioeconomic status, education, reading ability, and cognition. Journal of the American Geriatrics Society. 2011;59(8):1403–1411. [PMC free article: PMC3222272] [PubMed: 21797830]
- Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. New England Journal of Medicine. 2013;368(4):341–350. [PubMed: 23343063]
- Jorm AF, Rodgers B, Henderson AS, Korten AE, Jacomb PA, Christensen H, Mackinnon A. Occupation type as a predictor of cognitive decline and dementia in old age. Age and Ageing. 1998;27(4):477–483. [PubMed: 9884005]
- Kamegaya T, Araki Y, Kigure H, Yamaguchi H. Twelve-week physical and leisure activity programme improved cognitive function in community-dwelling elderly subjects: A randomized controlled trial. Psychogeriatrics. 2014;14(1):47–54. [PubMed: 24528600]
- Kang JH, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Archives of Internal Medicine. 2006;166(22):2462–2468. [PubMed: 17159011]
- Khalil N, Morrow LA, Needleman H, Talbott EO, Wilson JW, Cauley JA. Association of cumulative lead and neurocognitive function in an occupational cohort. Neuropsychology. 2009;23(1):10–19. [PubMed: 19210029]
- Koyama A, Houston DK, Simonsick EM, Lee JS, Ayonayon HN, Shahar DR, Rosano C, Satterfield S, Yaffe K. Association between the Mediterranean diet and cognitive decline in a biracial population. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2014;70(3):352–357. [PMC free article: PMC4351391] [PubMed: 24994847]
- Krahn D, Freese J, Hauser R, Barry K, Goodman B. Alcohol use and cognition at mid-life: The importance of adjusting for baseline cognitive ability and educational attainment. Alcoholism, Clinical and Experimental Research. 2003;27(7):1162–1166. [PubMed: 12878923]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. [PubMed: 10440369]
- Kremen WS, Lachman ME, Pruessner JC, Sliwinski M, Wilson RS. Mechanisms of age-related cognitive change and targets for intervention: Social interactions and stress. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2012;67(7):760–765. [PMC free article: PMC3391069] [PubMed: 22570134]
- Kuh D, Karunananthan S, Bergman H, Cooper R. A life-course approach to healthy ageing: Maintaining physical capability. Proceedings of the Nutrition Society. 2014;73(2):237–248. [PMC free article: PMC3981474] [PubMed: 24456831]
- Lautenschlager NT, Cox K, Cyarto EV. The influence of exercise on brain aging and dementia. Biochimica et Biophysica Acta. 2012;1822(3):474–481. [PubMed: 21810472]
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. [PMC free article: PMC3645500] [PubMed: 22818936]
- Levin ED, Timofeeva OA, Yang L, Petro A, Ryde IT, Wrench N, Seidler FJ, Slotkin TA. Early postnatal parathion exposure in rats causes sex-selective cognitive impairment and neurotransmitter defects which emerge in aging. Behavioural Brain Research. 2010;208(2):319–327. [PMC free article: PMC2831164] [PubMed: 20015457]
- Liu Y, Julkunen V, Paajanen T, Westman E, Wahlund LO, Aitken A, Sobow T, Mecocci P, Tsolaki M, Vellas B, Muehlboeck S, Spenger C, Lovestone S, Simmons A, Soininen H. Education increases reserve against Alzheimer's disease—Evidence from structural MRI analysis. Neuroradiology. 2012;54(9):929–938. [PMC free article: PMC3435513] [PubMed: 22246242]
- Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, Llewellyn DJ. Mediterranean diet, cognitive function, and dementia: A systematic review. Epidemiology. 2013;24(4):479–489. [PubMed: 23680940]
- Luanaigh CO, Lawlor BA. Loneliness and the health of older people. International Journal of Geriatric Psychiatry. 2008;23(12):1213–1221. [PubMed: 18537197]
- Madden DJ, Blumenthal JA, Allen PA, Emery CF. Improving aerobic capacity in healthy older adults does not necessarily lead to improved cognitive performance. Psychology and Aging. 1989;4(3):307–320. [PubMed: 2803624]
- Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. The Cochrane Database of Systematic Reviews. 2008;(4):Cd004514. [PubMed: 18843658]
- Manly JJ, Touradji P, Tang MX, Stern Y. Literacy and memory decline among ethnically diverse elders. Journal of Clinical and Experimental Neuropsychology. 2003;25(5):680–690. [PubMed: 12815505]
- Manly JJ, Schupf N, Tang MX, Stern Y. Cognitive decline and literacy among ethnically diverse elders. Journal of Geriatric Psychiatry and Neurology. 2005;18(4):213–217. [PubMed: 16306242]
- Martinez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvado J, San Julian B, Sanchez-Tainta A, Ros E, Valls-Pedret C, Martinez-Gonzalez MA. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. Journal of Neurology, Neurosurgery, and Psychiatry. 2013;84(12):1318–1325. [PubMed: 23670794]
- McEwen BS, Morrison JH. The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16–29. [PMC free article: PMC3753223] [PubMed: 23849196]
- Mehta KM, Simonsick EM, Rooks R, Newman AB, Pope SK, Rubin SM, Yaffe K. Black and white differences in cognitive function test scores: What explains the difference? Journal of the American Geriatrics Society. 2004;52(12):2120–2127. [PMC free article: PMC2939725] [PubMed: 15571554]
- Meng X, D'Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-anaylses and qualitative analyses. PLoS ONE. 2012;7(6):e38268. [PMC free article: PMC3366926] [PubMed: 22675535]
- Middleton LE, Barnes DE, Lui LY, Yaffe K. Physical activity over the life course and its association with cognitive performance and impairment in old age. Journal of the American Geriatric Society. 2010;58(7):1322–1326. [PMC free article: PMC3662219] [PubMed: 20609030]
- Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Annals of Internal Medicine. 2005;142(1):37–46. [PubMed: 15537682]
- Mortimer JA, Ding D, Borenstein AR, DeCarli C, Guo Q, Wu Y, Zhao Q, Chu S. Changes in brain volume and cognition in a randomized trial of exercise and social interaction in a community-based sample of non-demented Chinese elders. Journal of Alzheimer's Disease. 2012;30(4):757–766. [PMC free article: PMC3788823] [PubMed: 22451320]
- Muzet A. Environmental noise, sleep and health. Sleep Medicine Reviews. 2007;11(2):135–142. [PubMed: 17317241]
- NIH (National Institutes of Health). Multivitamin/mineral supplements. 2013. [January 8, 2015]. http://ods
.od.nih.gov /factsheets/MVMS-HealthProfessional. - NIH. What is the DASH eating plan? 2014a. [October 28, 2014]. http://www
.nhlbi.nih .gov/health/health-topics/topics/dash. - NIH. Exercise: Benefits of exercise. 2014b. [December 5, 2014]. www
.nihseniorhealth.gov /exerciseforolderadults /healthbenefits/01.html. - Nordling Nilson L, Barregard L, Sallsten G, Hagberg S. Self-reported symptoms and their effects on cognitive functioning in workers with past exposure to solvent-based glues: An 18-year follow-up. International Archives of Occupational and Environmental Health. 2007;81(1):69–79. [PubMed: 17410374]
- North A, Swant J, Salvatore MF, Gamble-George J, Prins P, Butler B, Mittal MK, Heltsley R, Clark JT, Khoshbouei H. Chronic methamphetamine exposure produces a delayed, long-lasting memory deficit. Synapse. 2013;67(5):245–257. [PMC free article: PMC3831527] [PubMed: 23280858]
- Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 2006;65(7):631–641. [PubMed: 16825950]
- OHTAC (Ontario Health Technology Advisory Committee). Vitamin B12 and cognitive function: OHTAC recommendation. Toronto: Queen's Printer for Ontario; 2013.
- O'Luanaigh C, O'Connell H, Chin AV, Hamilton F, Coen R, Walsh C, Walsh JB, Caokley D, Cunningham C, Lawlor BA. Loneliness and cognition in older people: The Dublin Healthy Ageing Study. Aging and Mental Health. 2012;16(3):347–352. [PubMed: 22129350]
- Pesonen AK, Raikkonen K, Heinonen K, Kajantie E, Forsen T, Eriksson JG. Depressive symptoms in adults separated from their parents as children: A natural experiment during World War II. American Journal of Epidemiology. 2007;166(10):1126–1133. [PubMed: 17875582]
- Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: A systematic review. Age and Ageing. 2008a;37(5):505–512. [PubMed: 18487267]
- Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatrics. 2008b;8(36) [PMC free article: PMC2642819] [PubMed: 19105840]
- Plassman BL, Williams JW Jr., Burke JR, Holsinger T, Benjamin S. Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Annals of Internal Medicine. 2010;153(3):182–193. [PubMed: 20547887]
- Querbes O, Aubry F, Pariente J, Lotterie JA, Demonet JF, Duret V, Puel M, Berry I, Fort JC, Celsis P. Early diagnosis of Alzheimer's disease using cortical thickness: Impact of cognitive reserve. Brain. 2009;132(Pt 8):2036–2047. [PMC free article: PMC2714060] [PubMed: 19439419]
- Rafnsson SB, Dilis V, Trichopoulou A. Antioxidant nutrients and age-related cognitive decline: A systematic review of population-based cohort studies. European Journal of Nutrition. 2013;52(6):1553–1567. [PubMed: 23744025]
- Rebok GW, Carlson MC, Glass TA, McGill S, Hill J, Wasik BA, Ialongo N, Frick KD, Fried LP, Rasmussen MD. Short-term impact of Experience Corps participation on children and schools: results from a pilot randomized trial. Journal of Urban Health. 2004;81(1):79–93. [PMC free article: PMC3456143] [PubMed: 15047787]
- Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, Widaman K, Hinton L, DeCarli C. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain. 2010;133(Pt 8):2196–2209. [PMC free article: PMC3139935] [PubMed: 20591858]
- Rickenbach EH, Almeida DM, Seeman TE, Lachman ME. Daily stress magnifies the association between cognitive decline and everyday memory problems: An integration of longitudinal and diary methods. Psychology and Aging. 2014;29(4):852–862. [PMC free article: PMC4268366] [PubMed: 25365691]
- Rikli RE, Edwards DJ. Effects of a three-year exercise program on motor function and cognitive processing speed in older women. Research Quarterly for Exercise and Sport. 1991;62(1):61–67. [PubMed: 2028094]
- Roerecke M, Rehm J. The cardioprotective association of average alcohol consumption and ischaemic heart disease: A systematic review and meta-analysis. Addiction. 2012;107(7):1246–1260. [PMC free article: PMC3348338] [PubMed: 22229788]
- Rosano C, Venkatraman VK, Guralnik J, Newman AB, Glynn NW, Launer L, Taylor CA, Williamson J, Studenski S, Pahor M, Aizenstein H. Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. Journal of Gerontology. 2010;65(6):639–647. [PMC free article: PMC2869531] [PubMed: 20348185]
- Rossom RC, Espeland MA, Manson JE, Dysken MW, Johnson KC, Lane DS, LeBlanc ES, Lederle FA, Masaki KH, Margolis KL. Calcium and vitamin D supplementation and cognitive impairment in the Women's Health Initiative. Journal of the American Geriatrics Society. 2012;60(12):2197–2205. [PMC free article: PMC3521077] [PubMed: 23176129]
- Ruscheweyh R, Willemer C, Kruger K, Duning T, Warnecke T, Sommer J, Volker K, Ho HV, Mooren F, Knecht S, Floel A. Physical activity and memory functions: An interventional study. Neurobiology of Aging. 2011;32(7):1304–1319. [PubMed: 19716631]
- Sabbath EL, Glymour MM, Berr C, Singh-Manoux A, Zins M, Goldberg M, Berkman LF. Occupational solvent exposure and cognition: Does the association vary by level of education? Neurology. 2012;78(22):1754–1760. [PMC free article: PMC3359584] [PubMed: 22641403]
- Sachs-Ericsson N, Blazer DG. Racial differences in cognitive decline in a sample of community-dwelling older adults: the mediating role of education and literacy. American Journal of Geriatric Psychiatry. 2005;13(11):968–975. [PubMed: 16286440]
- Salanova M, Llorens S, Cifre E. The dark side of technologies: Technostress among users of information and communication technologies. International Journal of Psychology. 2013;48(3):422–436. [PubMed: 22731610]
- Samieri C, Grodstein F, Rosner BA, Kang JH, Cook NR, Manson JE, Buring JE, Willett WC, Okereke OI. Mediterranean diet and cognitive function in older age: Results from the Women's Health Study. Epidemiology. 2013;24(4):490–499. [PMC free article: PMC3674216] [PubMed: 23676264]
- Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302(6):627–637. [PMC free article: PMC2765045] [PubMed: 19671904]
- Schaie KW. Intellectual development in adulthood: The Seattle Longitudinal Study. New York: Cambridge University Press; 1996.
- Schneider AL, Lutsey PL, Alonso A, Gottesman RF, Sharrett AR, Carson KA, Gross M, Post WS, Knopman DS, Mosley TH, Michos ED. Vitamin D and cognitive function and dementia risk in a biracial cohort: The ARIC Brain MRI Study. European Journal of Neurology. 2014;21(9):1211–1218. e1269-e1270. [PMC free article: PMC4114998] [PubMed: 24846449]
- Seeman TE, Miller-Martinez DM, Stein Merkin S, Lachman ME, Tun PA, Karlamangla AS. Histories of social engagement and adult cognition: Midlife in the U.S. Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2011;66(Suppl 1):i141–i152. [PMC free article: PMC3132769] [PubMed: 21196438]
- Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, Newby DE, Mills NL. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet. 2013;382(9897):1039–1048. [PMC free article: PMC3809511] [PubMed: 23849322]
- Shankar A, Hamer M, McMunn A, Steptoe A. Social isolation and loneliness: Relationships with cognitive function during 4 years of follow-up in the English Longitudinal Study of Ageing. Psychosomatic Medicine. 2013;75(2):161–170. [PubMed: 23362501]
- Singh-Manoux A, Marmot MG, Glymour M, Sabia S, Kivimaki M, Dugravot A. Does cognitive reserve shape cognitive decline? Annals of Neurology. 2011;70(2):296–304. [PMC free article: PMC3152621] [PubMed: 21563209]
- Slinin Y, Paudel M, Taylor BC, Ishani A, Rossom R, Yaffe K, Blackwell T, Lui LY, Hochberg M, Ensrud KE. Association between serum 25(OH) vitamin D and the risk of cognitive decline in older women. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2012;67(10):1092–1098. [PMC free article: PMC3437964] [PubMed: 22454371]
- Small BJ, Dixon RA, McArdle JJ, Grimm KJ. Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. Neuropsychology. 2012;26(2):144–155. [PMC free article: PMC3761970] [PubMed: 22149165]
- Smith PJ, Blumenthal JA, Babyak MA, Craighead L, Welsh-Bohmer KA, Browndyke JN, Strauman TA, Sherwood A. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010a;55(6):1331–1338. [PMC free article: PMC2974436] [PubMed: 20305128]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine. 2010b;72(3):239–252. [PMC free article: PMC2897704] [PubMed: 20223924]
- Sole-Padulles C, Bartres-Faz D, Junque C, Vendrell P, Rami L, Clemente IC, Bosch B, Villar A, Bargallo N, Jurado MA, Barrios M, Molinuevo JL. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer's disease. Neurobiology of Aging. 2009;30(7):1114–1124. [PubMed: 18053618]
- Spirduso WW. Reaction and movement time as a function of age and physical activity level. Journal of Gerontology. 1975;30(4):435–440. [PubMed: 1141674]
- Spirduso WW, Clifford P. Replication of age and physical activity effects on reaction and movement time. Journal of Gerontology. 1978;33(1):26–30. [PubMed: 618962]
- Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(15):5797–5801. [PMC free article: PMC3625264] [PubMed: 23530191]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. [PMC free article: PMC2739591] [PubMed: 19467352]
- Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurology. 2012;11(11):1006–1012. [PMC free article: PMC3507991] [PubMed: 23079557]
- Stine-Morrow EA, Parisi JM, Morrow DG, Park DC. The effects of an engaged lifestyle on cognitive vitality: A field experiment. Psychology and Aging. 2008;23(4):778–786. [PMC free article: PMC2774272] [PubMed: 19140649]
- Stine-Morrow EA, Payne BR, Roberts BW, Kramer AF, Morrow DG, Payne L, Hill PI, Jackson JJ, Gao X, Noh SR, Janke MC. Training versus engagement as paths to cognitive enrichment with aging. Psychology and Aging. 2014;29(4):891–906. [PMC free article: PMC4361254] [PubMed: 25402337]
- Szabo S, Tache Y, Somogyi A. The legacy of Hans Selye and the origins of stress research: A retrospective 75 years after his landmark brief “letter” to the editor of Nature. Stress. 2012;15(5):472–478. [PubMed: 22845714]
- Terrett G, McLennan SN, Henry JD, Biernacki K, Mercuri K, Curran HV, Rendell PG. Prospective memory impairment in long-term opiate users. Psychopharmacology. 2014;231(13):2623–2632. [PubMed: 24448901]
- Thames AD, Arbid N, Sayegh P. Cannabis use and neurocognitive functioning in a non-clinical sample of users. Addictive Behaviors. 2014;39(5):994–999. [PMC free article: PMC4032061] [PubMed: 24556155]
- Thomas PA. Gender, social engagement, and limitations in late life. Social Science and Medicine. 2011;73(9):1428–1435. [PubMed: 21906863]
- Thomee S, Dellve L, Harenstam A, Hagberg M. Perceived connections between information and communication technology use and mental symptoms among young adults: A qualitative study. BMC Public Health. 2010;10:66. [PMC free article: PMC2836296] [PubMed: 20152023]
- Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2004;59(3):268–274. [PubMed: 15031312]
- Titova OE, Ax E, Brooks SJ, Sjogren P, Cederholm T, Kilander L, Kullberg J, Larsson EM, Johansson L, Ahlstrom H, Lind L, Schioth HB, Benedict C. Mediterranean diet habits in older individuals: Associations with cognitive functioning and brain volumes. Experimental Gerontology. 2013;48(12):1443–1448. [PubMed: 24126083]
- Tschanz JT, Pfister R, Wanzek J, Corcoran C, Smith K, Tschanz BT, Steffens DC, Ostbye T, Welsh-Bohmer KA, Norton MC. Stressful life events and cognitive decline in late life: Moderation by education and age. The Cache County Study. International Journal of Geriatric Psychiatry. 2013;28(8):821–830. [PMC free article: PMC3706540] [PubMed: 23037866]
- Tun PA, Miller-Martinez D, Lachman ME, Seeman T. Social strain and executive function across the lifespan: The dark (and light) sides of social engagement. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition. 2013;20(3):320–338. [PMC free article: PMC3508192] [PubMed: 22873285]
- USDA (U.S. Department of Agriculture). Dietary guidelines for Americans. 2014. [October 28, 2014]. http://www
.cnpp.usda .gov/DietaryGuidelines.htm. - van Holst RJ, Schilt T. Drug-related decrease in neuropsychological functions of abstinent drug users. Current Drug Abuse Reviews. 2011;4(1):42–56. [PubMed: 21466500]
- Vaynman S, Gomez-Pinilla F. License to run: Exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabilitation and Neural Repair. 2005;19(4):283–295. [PubMed: 16263961]
- Vemuri P, Lesnick TG, Przybelski SA, Machulda M, Knopman DS, Mielke MM, Roberts RO, Geda YE, Rocca WA, Petersen RC, Jack CR. Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurology. 2014;71(8):1017–1024. [PMC free article: PMC4266551] [PubMed: 25054282]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, Wojcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, Kramer AF. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Frontiers in Aging Neuroscience. 2010;2 [PMC free article: PMC2947936] [PubMed: 20890449]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, Szabo A, Phillips SM, Wojcicki TR, Mailey EL, Olson EA, Gothe N, Vieira-Potter VJ, Martin SA, Pence BD, Cook MD, Woods JA, McAuley E, Kramer AF. Neurobiological markers of exercise-related brain plasticity in older adults. Brain, Behavior, and Immunity. 2013a;28:90–99. [PMC free article: PMC3544982] [PubMed: 23123199]
- Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Science. 2013b;17(10):525–544. [PMC free article: PMC4565723] [PubMed: 24029446]
- Wald DS, Kasturiratne A, Simmonds M. Effect of folic acid, with or without other B vitamins, on cognitive decline: Meta-analysis of randomized trials. The American Journal of Medicine. 2010;123(6):522–527. [PubMed: 20569758]
- Wang GY, Wouldes TA, Russell BR. Methadone maintenance treatment and cognitive function: A systematic review. Current Drug Abuse Reviews. 2013;6(3):220–230. [PubMed: 23773088]
- Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran C, Tschanz JT, Norton MC, Welsh-Bohmer KA. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: The Cache County Study on Memory, Health and Aging. American Journal of Clinical Nutrition. 2013;98(5):1263–1271. [PMC free article: PMC3798079] [PubMed: 24047922]
- Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911–1920. [PubMed: 17596582]
- Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81(4):314–321. [PMC free article: PMC3772831] [PubMed: 23825173]
- Wilson VK, Houston DK, Kilpatrick L, Lovato J, Yaffe K, Cauley JA, Harris TB, Simonsick EM, Ayonayon HN, Kritchevsky SB, Sink KM. Relationship between 25-hydroxyvitamin D and cognitive function in older adults: The Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2014;62(4):636–641. [PMC free article: PMC3989387] [PubMed: 24635412]
- Wong HS, Edwards P. Nature or nurture: A systematic review of the effect of socio-economic status on the developmental and cognitive outcomes of children born preterm. Maternal and Child Health Journal. 2013;17(9):1689–1700. [PubMed: 23135625]
- Wu LT, Blazer DG. Illicit and nonmedical drug use among older adults: A review. Journal of Aging and Health. 2011;23(3):481–504. [PMC free article: PMC3097242] [PubMed: 21084724]
- Wu LT, Gersing KR, Swartz MS, Burchett B, Li TK, Blazer DG. Using electronic health records data to assess comorbidities of substance use and psychiatric diagnoses and treatment settings among adults. Journal of Psychiatric Research. 2013;47(4):555–563. [PMC free article: PMC3581730] [PubMed: 23337131]
- Zahodne LB, Glymour MM, Sparks C, Bontempo D, Dixon RA, MacDonald SW, Manly JJ. Education does not slow cognitive decline with aging: 12-year evidence from the Victoria Longitudinal Study. Journal of the International Neuropsychological Society. 2011;17(6):1039–1046. [PMC free article: PMC3285821] [PubMed: 21923980]
- Zhu N, Jacobs DR Jr., Schreiner PJ, Yaffe K, Bryan N, Launer LJ, Whitmer RA, Sidney S, Demerath E, Thomas W, Bouchard C, He K, Reis J, Sternfeld B. Cardiorespiratory fitness and cognitive function in middle age: The CARDIA study. Neurology. 2014;82(15):1339–1346. [PMC free article: PMC4001190] [PubMed: 24696506]
Footnotes
- 1
These tasks included the critical flicker fusion test (the point at which the viewer perceives a flickering light source is a steady, continuous one, used as a measure of the brain's processing capacity), the digit symbol substitution test (which measures memory and processing speed), and the Stroop test (which also tests memory, processing, and executive brain functions) (Cohn et al., 1984; Rikli and Edwards, 1991).
- 2
The Experience Corps trains and places volunteers in elementary schools for an academic year and covers three areas: general literacy support, library support, and conflict resolution. It is designed to bolster memory and executive functions of the volunteers by reading with children, problem solving, and working with team members and through various program activities (Rebok et al., 2004).
- Risk and Protective Factors and Interventions: Lifestyle and Physical Environmen...Risk and Protective Factors and Interventions: Lifestyle and Physical Environment - Cognitive Aging
Your browsing activity is empty.
Activity recording is turned off.
See more...