NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
| Chemical name: | 99mTc-Labeled acetylated, 2,3,5-triiodobenzoic acid- and diethylenetriamine pentaacetic acid-conjugated, and PEGylated ethylenediamine-core generation 4 polyamidoamine dendrimers |
|
| Abbreviated name: | 99mTc-G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] | |
| Synonym: | ||
| Agent Category: | Compounds | |
| Target: | Non-targeted | |
| Target Category: | Non-targeted | |
| Method of detection: | Multimodality imaging (SPECT and CT) | |
| Source of signal / contrast: | 99mTc and iodine (2,3,5-triiodobenzoic acid (TIBA)) | |
| Activation: | No | |
| Studies: |
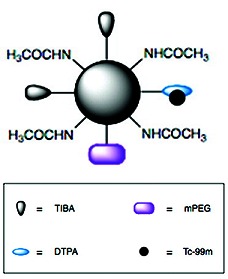
| Schematic structure of 99mTc-G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] by Criscione et al. (1). |
Background
[PubMed]
99mTc-Labeled acetylated, 2,3,5-triiodobenzoic acid (TIBA)- and diethylenetriamine pentaacetic acid (DTPA)-conjugated, and PEGylated ethylenediamine-core generation 4 polyamidoamine dendrimers (G4 PAMAM), abbreviated as 99mTc-G4-[[[[Ac]-TIBA]-DTPA]-mPEG12], is a blood pool multimodal agent synthesized by Criscione et al. for single-photon emission computed tomography (SPECT)/computed tomography (2) (1).
In the recent years, the fact that imaging modalities with high sensitivity have relatively poor resolution, while those with high resolution have relatively poor sensitivity, has triggered the integration of multiple modalities and the use of hybrid instrument technology in imaging (3, 4). This has also boosted the development of multimodal imaging agents, which further enhances the benefits of hybrid technology, allowing better characterization of diseases and disease processes (5-7). However, it is still challenging to incorporate enough labels for detection by the relatively low-sensitive modalities and to select an appropriate radionuclide in a radiotracer-based imaging approach in the development of multimodal agents (1).
Criscione et al. synthesized a radiolabeled, multimodal contrast agent, 99mTc-G4-[[[[Ac]-TIBA]-DTPA]-mPEG12], for preclinical microSPECT/CT imaging (1). The design of this agent is based on the G4 PAMAM dendrimers, which were used as the core structure of the multimodal agent. Dendrimers have been selected for two main reasons. First, their well-defined, multifunctional surface (64 primary amines) offers the ability to conjugate several different moieties with negligible steric hindrance. Second, dendrimer-based contrast agents have already been successfully designed and examined with different imaging modalities (1, 8, 9). However, PAMAM dendrimers have a highly cationic surface, which limits their water solubility and can cause hemolysis in humans (1). Dendrimers also have a relatively short circulation time when they are used for developing blood pool imaging agents. To overcome these problems, Criscione et al. partially acetylated the dendrimer surface to reduce the positive surface charge and PEGylated the remaining surface amines to enhance the circulation time and limit the clearance by the reticuloendothelial system (1). These modifications provided dendrimers the desired water solubility, long intravascular residence time, and predominant renal clearance (1). To induce the dendrimer construct's multimodal capability, Criscione et al. selected TIBA and 99mTc-DTPA for X-ray attenuation and radioactive signal, respectively (1). TIBA possesses X-ray attenuation properties similar to those of the clinically used, small-molecule triiodobenzoic acid derivative Omnipaque 350. The studies by Criscione et al. have also shown that sufficient iodine weight percent for effective X-ray attenuation without sacrificing aqueous solubility can be achieved by conjugating TIBA to dendrimers (1). The acyclic chelator DTPA was used to chelate tin(II)-reduced 99mTc because of its established chemistry and stability. In vitro and animal studies have confirmed the potential usefulness of this multimodal agent in the quantification of intramyocardial blood volume and blood flow (1).
Related Resource Links:
Synthesis
[PubMed]
Criscione et al. described the synthesis of 99mTc-G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] in detail (1). The synthesis could be roughly divided into five steps. The G4 dendrimers were partially acetylated with sulfosuccinimidyl acetate to generate acetylated dendrimers (G4-[Ac]). Then TIBA was covalently conjugated to G4-[Ac] through the surface amines to generate G4-[[Ac]-TIBA]. To chelate the nuclear probe 99mTc, 1-(4-isothiocyanatobenzyl)diethylenetriamine pentaacetic acid (p-SCN-Bn-DTPA) was coupled to the primary surface amines of the dendrimer via isothiocyanate linkage. This step resulted in the product G4-[[[Ac]-TIBA]-DTPA]. G4-[[[Ac]-TIBA]-DTPA] was subsequently PEGylated with succinimidyl-(N-methyl-dodecaethylene glycol)ester (mPEG12) to generate the G4-[[[[Ac]-TIBA]-DTPA]-mPEG12]. The chemical yields of the intermediate products were 97% for G4-[Ac], 82% for G4-[[Ac]-TIBA], 89% for G4-[[[Ac]-TIBA]-DTPA], and 83% for G4-[[[[Ac]-TIBA]-DTPA]-mPEG12]. Finally, 99mTc-labeling was completed with the reaction of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] (50 mg for imaging purposes and 20 mg for biodistribution studies) and 99mTcO4– (18.5 MBq (500 μCi)) for 2 min (for imaging purposes) or for 10 min (for biodistribution studies) at 25ºC. The reaction mixture was directly used for studies without further purification. The radiochemical purity was >80%.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
Criscione et al. characterized the intermediate products with 1H nuclear magnetic resonance (NMR) spectroscopy and dynamic light scattering (1). The results showed that G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] had 3 remaining primary amines, 35 coupled acetyl groups, ~15 TIBA moieties, 4 DTPA, and 7 mPEG12. The hydrodynamic diameter and molecular weight of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] were 12.37 ± 6.09 nm and 19,762, respectively.
The X-ray attenuation property of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] was analyzed with serially diluted G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] in deionized water using microCT imaging (1). A linear attenuation versus the iodine concentration curve, an essential criterion for X-ray contrast agents, was obtained for G4-[[[[Ac]-TIBA]-DTPA]-mPEG12]. The extrapolated iodine concentrations in G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] were consistent with the estimated iodine weight percent (~19% wt) determined with 1H NMR.
Animal Studies
Rodents
[PubMed]
The biodistribution and pharmacokinetics of 99mTc-G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] were evaluated in C57BL/6 mice after injection of 4.255 MBq (115 μCi) of the agent. Mice were euthanized at 0.5, 1, 2, 4, and 6 h after injection (n = 4 mice/time point) (1). Blood samples and organs were harvested, and radioactivity was counted. The analysis showed that the agent’s half-life was 0.72 h (43 min), and the agent was cleared from the blood pool predominantly by the kidneys.
The efficacy of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] as a blood pool CT contrast agent was assessed in C57BL/6 mice with serial cardiac microCT after intravenous injection of either 1.27 µmol (n = 2 mice) or 2.53 µmol (n = 2 mice) of the agent (1). Intravascular and intraventricular enhancement with an excellent contrast/noise ratio in the right and left ventricles of the heart was observed with both doses. The enhancement persisted for >90 min after injection.
The multimodal capability of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] was assessed in mice euthanized 5 min after injection of 16.28 ± 0.96 MBq (440 ± 26 μCi) 99mTc-G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] (n = 5 mice) (1). Post-mortem microSPECT/CT imaging revealed intravascular retention, minimal liver accumulation, and substantial colocalization of the radioactivity and the dendritic iodinated contrast within the intravascular and intraventricular cavities in the absence of motion artifacts. These results demonstrate the potential usefulness of the agent as a blood pool multimodal agent and also provide evidence for the formation of a sufficiently pure and stable chelate, as either colloidal or free hydrolyzed/reduced 99mTc would be cleared predominantly by the liver.
Human Studies
[PubMed]
No references are currently available.
References
- 1.
- Criscione J.M. et al. Development and application of a multimodal contrast agent for SPECT/CT hybrid imaging. Bioconjug Chem. 2011;22(9):1784–92. [PMC free article: PMC3204385] [PubMed: 21851119]
- 2.
- Hoekzema E. et al. [(11)C]-DASB microPET imaging in the aged rat: frontal and meso-thalamic increases in serotonin transporter binding. Exp Gerontol. 2011;46(12):1020–5. [PubMed: 21983171]
- 3.
- Cherry S.R. Multimodality in vivo imaging systems: twice the power or double the trouble? Annu Rev Biomed Eng. 2006;8:35–62. [PubMed: 16834551]
- 4.
- Cherry S.R. Multimodality imaging: beyond PET/CT and SPECT/CT. Semin Nucl Med. 2009;39(5):348–53. [PMC free article: PMC2735449] [PubMed: 19646559]
- 5.
- Lee S., Chen X. Dual-modality probes for in vivo molecular imaging. Mol Imaging. 2009;8(2):87–100. [PubMed: 19397854]
- 6.
- Louie A. Multimodality imaging probes: design and challenges. Chem Rev. 2010;110(5):3146–95. [PMC free article: PMC2878382] [PubMed: 20225900]
- 7.
- Jennings L.E., Long N.J. 'Two is better than one'--probes for dual-modality molecular imaging. Chem Commun (Camb). 2009;(24):3511–24. [PubMed: 19521594]
- 8.
- Mintzer M.A., Grinstaff M.W. Biomedical applications of dendrimers: a tutorial. Chem Soc Rev. 2011;40(1):173–90. [PubMed: 20877875]
- 9.
- Cheng Y. et al. Design of biocompatible dendrimers for cancer diagnosis and therapy: current status and future perspectives. Chem Soc Rev. 2011;40(5):2673–703. [PubMed: 21286593]
- Review (99m)Tc-Labeled acetylated dendrimer poly(amido)-amine generation 5-PEGylated folic acid-2-(p-isothiocyanatobenzyl)-6-methyl-diethylenetriamine pentaacetic acid conjugate.[Molecular Imaging and Contrast...]Review (99m)Tc-Labeled acetylated dendrimer poly(amido)-amine generation 5-PEGylated folic acid-2-(p-isothiocyanatobenzyl)-6-methyl-diethylenetriamine pentaacetic acid conjugate.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (99m)Tc-Labeled acetylated dendrimer poly(amido)-amine generation 5-folic acid-2-(p-isothiocyanatobenzyl)-6-methyl-diethylenetriamine pentaacetic acid conjugate.[Molecular Imaging and Contrast...]Review (99m)Tc-Labeled acetylated dendrimer poly(amido)-amine generation 5-folic acid-2-(p-isothiocyanatobenzyl)-6-methyl-diethylenetriamine pentaacetic acid conjugate.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Development and application of a multimodal contrast agent for SPECT/CT hybrid imaging.[Bioconjug Chem. 2011]Development and application of a multimodal contrast agent for SPECT/CT hybrid imaging.Criscione JM, Dobrucki LW, Zhuang ZW, Papademetris X, Simons M, Sinusas AJ, Fahmy TM. Bioconjug Chem. 2011 Sep 21; 22(9):1784-92. Epub 2011 Sep 6.
- Design of (99m)Tc-Labeled Low Generation Dendrimer-Entrapped Gold Nanoparticles for Targeted Single Photon Emission Computed Tomography/Computed Tomography Imaging of Gliomas.[J Biomed Nanotechnol. 2019]Design of (99m)Tc-Labeled Low Generation Dendrimer-Entrapped Gold Nanoparticles for Targeted Single Photon Emission Computed Tomography/Computed Tomography Imaging of Gliomas.Li Y, Zhao L, Xu X, Sun N, Qiao W, Xing Y, Shen M, Zhu M, Shi X, Zhao J. J Biomed Nanotechnol. 2019 Jun 1; 15(6):1201-1212.
- (99m)Tc-Labeled Multifunctional Low-Generation Dendrimer-Entrapped Gold Nanoparticles for Targeted SPECT/CT Dual-Mode Imaging of Tumors.[ACS Appl Mater Interfaces. 2016](99m)Tc-Labeled Multifunctional Low-Generation Dendrimer-Entrapped Gold Nanoparticles for Targeted SPECT/CT Dual-Mode Imaging of Tumors.Li X, Xiong Z, Xu X, Luo Y, Peng C, Shen M, Shi X. ACS Appl Mater Interfaces. 2016 Aug 10; 8(31):19883-91. Epub 2016 Jul 27.
- 99mTc-Labeled acetylated, 2,3,5-triiodobenzoic acid- and diethylenetriamine pent...99mTc-Labeled acetylated, 2,3,5-triiodobenzoic acid- and diethylenetriamine pentaacetic acid-conjugated, and PEGylated ethylenediamine-core generation 4 polyamidoamine dendrimers - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro