NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Trimipramine is a tricyclic antidepressant used in the therapy of major (endogenous) as well as reactive (exogenous) depression. In clinical trials, trimipramine therapy was not associated with an increased rate of elevations in serum aminotransferase levels, and it has yet to be linked to instances of clinically apparent acute liver injury.
Background
Trimipramine (trye mip' ra meen) is a tricyclic antidepressant that is believed to act by enhancing serotonergic and dopaminergic neurotransmission. Like most tricyclic antidepressants, trimipramine is a weak inhibitor of serotonin, norepinephrine and dopamine reuptake, but also has direct antagonist activity for some serotonin and adrenergic receptors. Trimipramine has been shown to alleviate symptoms of depression, both in patients with neurotic or situation depression as well as those with major, endogenous depression. Trimipramine was approved for use in the United States in 1979 and is still clinically available, although now not widely used. Trimipramine is available as capsules of 25, 50 and 100 mg generically and under the brand name Surmontil. The typical initial dosage in adults is 75 mg daily in divided doses, which can be modified to once daily and increased in total dose based upon efficacy and tolerance to as highly as 150 to 200 mg once daily. Common side effects are diarrhea, nausea, vomiting, fatigue, drowsiness, headache, insomnia, weight gain and sexual dysfunction. Rare potential side effects include increased suicide risk, cardiac arrhythmias, urinary retention and acute serotonin syndrome.
Hepatotoxicity
In clinical trials, liver test abnormalities were uncommon in patients taking trimipramine (<1%) and generally no more frequent than in placebo or comparator arm recipients. No instances of acute, clinically apparent liver injury attributed to trimipramine have been reported. Most other tricyclic antidepressants in clinical use have been associated with occasional instances of acute liver injury, usually arising within 2 to 8 weeks of starting therapy. The pattern of serum enzyme elevations varied from hepatocellular to cholestatic and autoimmune features were uncommon. Immunoallergic features (rash, fever, eosinophilia) can occur, but are not prominent. Most cases of acute liver injury due to tricyclic antidepressants are mild-to-moderate in severity and resolve within one to three months. Acute liver failure due to tricyclic antidepressants has been described, but is very rare. No such cases have been linked to trimipramine use but it is rarely used.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which trimipramine might cause liver injury is not known. Trimipramine is metabolized at least in part by the liver, but it has not been linked to significant drug-drug interactions.
Outcome and Management
The serum aminotransferase elevations that occur on amoxapine therapy are usually self-limited and do not require dose modification or discontinuation of therapy. No instances of acute liver failure or vanishing bile duct syndrome due to trimipramine have been reported. There is no information on cross sensitivity to liver injury between trimipramine and other tricyclic antidepressants, but switching to another class of agents (such as the selective serotonin reuptake inhibitors) is probably prudent.
Drug Class: Antidepressant Agents
Other Drugs in the Subclass, Tricyclics: Amitriptyline, Amoxapine, Clomipramine, Desipramine, Doxepin, Imipramine, Nortriptyline, Protriptyline
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Trimipramine – Generic, Surmontil®
DRUG CLASS
Antidepressant Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
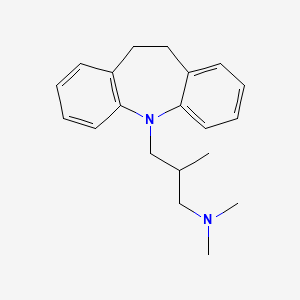
| Trimipramine | 739-71-9 | C20-H26-N2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 05 April 2020
Abbreviations: MAO inhibitor, monoamine oxidase inhibitor; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin and norepinephrine reuptake inhibitor.
- Zimmerman HJ. Antidepressants. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 493-8.(Expert review of hepatotoxicity published in 1999, trimipramine is listed as a tricyclic antidepressant with a very low incidence of hepatotoxicity).
- Larrey D. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 2nd ed. New York: Informa Healthcare USA, 2007, pp. 443-62.(Review of hepatotoxicity published in 2007; mentions that trimipramine, like other imipramine antidepressants, has been implicated in rare cases of acute hepatitis).
- O'Donnell JM, Bies RR, Shelton RC. Drug therapy of depression and anxiety disorders. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 267-77.(Textbook of pharmacology and therapeutics).
- Salzmann MM. A controlled trial with trimipramine, a new anti-depressant drug. Br J Psychiatry. 1965;111:1105–6. [PubMed: 5320547](Among 27 depressed patients treated with either imipramine or trimipramine for an unstated duration, response rates were better with trimipramine; no description of side effects was provided).
- Burke BV, Sainsbury MJ, Mezo BA. A comparative trial of amitriptyline and trimipramine in the treatment of depression. Med J Aust. 1967;1:1216–8. [PubMed: 5339123](Among 26 patients with severe depression treated with either amitriptyline or trimipramine for 4 weeks, only 19 could complete the study and efficacy and side effects were similar with the two agents; no mention of ALT elevations or hepatotoxicity).
- Young JP, Lader MH, Hughes WC. Controlled trial of trimipramine, monoamine oxidase inhibitors, and combined treatment in depressed outpatients. Br Med J. 1979;2(6201):1315–7. [PMC free article: PMC1597363] [PubMed: 391342](Among 135 patients with depression treated with trimipramine, an MAO inhibitor or both for 6 weeks, trimipramine alone was more effective than the combination or the MAO inhibitor alone; side effects were not specifically addressed and no mention of ALT elevations or hepatotoxicity).
- Settle EC Jr, Ayd FJ Jr. Trimipramine: twenty years' worldwide clinical experience. J Clin Psychiatry. 1980;41:266–74. [PubMed: 7400104](Review of the 20 year clinical experience with trimipramine [largely in Europe] before its approval in the US in 1979 mentions that side effects are similar to those with other tricyclics, the most common being sedation, dry mouth, tremor, headaches and, more rarely, excitation and agitation; no mention of ALT elevations or hepatotoxicity).
- Rifkin A, Saraf K, Kane J, Ross D, Klein DF. A comparison of trimipramine and imipramine: a controlled study. J Clin Psychiatry. 1980;41:124–9. [PubMed: 7364735](Among 38 hospitalized patients with depression treated with trimipramine or imipramine for 3 weeks, clinical efficacy was similar, but side effects were less with trimipramine; no mention of ALT elevations or hepatotoxicity; 10 patients did not complete the study due to "uncooperativeness", clinical deterioration or adverse reactions).
- Assalian P, Rosengarten MD, Phillips R. A comparative trial of the antidepressant, anxiolytic, and cardiovascular effects of trimipramine and doxepin in depressed hospitalized patients. J Clin Psychiatry. 1985;46:90–4. [PubMed: 2857709](Among 25 hospitalized patients with depression treated with trimipramine or doxepin for 4 weeks, results of monitoring of laboratory tests were not provided).
- Cournoyer G, de Montigny C, Ouellette J, Langlois R, Elie R, Caille G, le Morvan P. A comparative double-blind controlled study of trimipramine and amitriptyline in major depression: lack of correlation with 5-hydroxytryptamine reuptake blockade. J Clin Psychopharmacol. 1987;7:385–93. [PubMed: 3323262](Among 34 patients with major depression treated with trimipramine or amitriptyline for 21 days, routine laboratory test results did not change in either group).
- Cassidy S, Henry J. Fatal toxicity of antidepressant drugs in overdose. Br Med J (Clin Res Ed) 1987; 295(6605): 1021-4. Erratum in Br Med J (Clin Res Ed) 1987; 295(6610): 1382. [PMC free article: PMC1248068] [PubMed: 3690249](Analysis of prescription rates and overdose fatality reports in the UK between 1975 and 1984 found the highest fatal toxicity indices for tricyclic antidepressants [38 deaths per million] compared to MAO inhibitors [27], with high rates of amitriptyline [46], nortriptyline [39], imipramine [28] and trimipramine [28]).
- Lapierre YD. A review of trimipramine. 30 years of clinical use. Drugs. 1989;38 Suppl 1:17–24. [PubMed: 2693051](Summary of more than 25 trials of trimipramine in comparison to placebo and other antidepressants found the side effect profile similar to other tricyclic antidepressants; no mention of ALT elevations or hepatotoxicity).
- Bender S, Olbrich HM, Fischer W, Hornstein C, Schoene W, Falkai P, Haarmann C, et al. Trimipramine Study Group. Antipsychotic efficacy of the antidepressant trimipramine: a randomized, double-blind comparison with the phenothiazine perazine. Pharmacopsychiatry. 2003;36:61–9. [PubMed: 12734763](Among 95 patients with acute schizophrenia treated with trimipramine or perazine for 36 days, the antipsychotic effect of the phenothiazine was greater; no mention of changes in ALT levels or hepatotoxicity).
- Wehmeier PM, Kluge M, Maras A, Riemann D, Berger M, Kohnen R, Dittmann RW, et al. Fluoxetine versus trimipramine in the treatment of depression in geriatric patients. Pharmacopsychiatry. 2005;38:13–6. [PubMed: 15706460](Among 41 elderly patients with major depression treated with trimipramine or fluoxetine for 6 weeks, both efficacy and side effects were similar; one patient on trimipramine developed abnormal ALT levels, but specifics and outcome were not provided).
- Gutscher K. Rauber-Lü, Haller M, Braun M, Kupferschmidt H, Kullak-Ublick GA, Ceschi A. Patterns of toxicity and factors influencing severity in acute adult trimipramine poisoning. Br J Clin Pharmacol. 2013;75:227–35. [PMC free article: PMC3555062] [PubMed: 22642681](Over an 18 year period, 230 cases of suspected trimipramine overdose were reported to a Swiss Toxicology Registry, mostly due to suicide attempt, symptoms being somnolence [61%], tachycardia [19%], coma [18%] and convulsions [6%], with fatal outcome in 3 patients [8.4-25 g doses] caused by cardiovascular collapse; no mention of liver manifestations or hepatic failure).
- Lucena MI, Carvajal A, Andrade RJ, Velasco A. Antidepressant-induced hepatotoxicity. Expert Opin Drug Saf. 2003;2:249–62. [PubMed: 12904104](Review of hepatotoxicity of antidepressants; antidepressant use has increased markedly between 1992 and 2002, accounting for 5% of cases of hepatotoxicity; SSRIs are less likely to cause injury than tricyclics and MAO inhibitors; trimipramine is not mentioned).
- Degner D, Grohmann R, Kropp S. RüE, Bender S, Engel RR, Schmidt LG. Severe adverse drug reactions of antidepressants: results of the German multicenter drug surveillance program AMSP. Pharmacopsychiatry. 2004;37 Suppl 1:S39–45. [PubMed: 15052513](Analysis of adverse drug reactions reported from 1993-2000 in 35 psychiatric hospitals; 0.3% of trimipramine recipients had a severe adverse event which was hepatic in approximately one-third, these rates being similar to the overall rates of tricyclics).
- DeSanty KP, Amabile CM. Antidepressant-induced liver injury. Ann Pharmacother. 2007;41:1201–11. [PubMed: 17609231](Review of drug induced liver injury and reports of injury from MAO inhibitors, SSRIs, tricyclics and atypical agents; no specific discussion of trimipramine).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including 4 from psychotropic agents [quetiapine, nefazodone, fluoxetine, venlafaxine], but none were linked to tricyclic antidepressants).
- Park SH, Ishino R. Liver injury associated with antidepressants. Curr Drug Saf. 2013;8:207–23. [PubMed: 23914755](Review of drug induced liver injury due to antidepressants including SSRIs; trimipramine is not discussed).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, one being attributed to an SSRI [venlafaxine], but none to a tricyclic antidepressant).
- Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry. 2014;171:404–15. [PubMed: 24362450](Review of the frequency and clinical features of drug induced liver injury due to antidepressants; imipramine, desipramine and amitriptyline are discussed, but not trimipramine).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, only one of which was attributed to an antidepressant [amitriptyline] and none to a MAO inhibitor, SSRI or SNRI).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 20 cases [2%] were attributed to antidepressants including 9 due to SNRIs [7 to duloxetine, 1 each to nefazodone and trazodone], 5 to bupropion, 5 to SSRIs [3 to escitalopram, and 1 each to fluoxetine and sertraline], and only 1 to tricyclics [imipramine], but none to trimipramine).
- Woo HJ, Kim HY, Choi ES, Cho YH, Kim Y, Lee JH, Jang E. Drug-induced liver injury: A 2-year retrospective study of 1169 hospitalized patients in a single medical center. Phytomedicine. 2015;22:1201–5. [PubMed: 26598920](Among 1169 inpatients seen at a single Korean referral medical center over a 2 year period, 11 developed suspected drug induced liver injury, 6 attributed to dietary supplements and 5 to conventional drugs including 2 antidepressants [minocycline, donepezil, warfarin, gabapentin/milnacipran, and antihistamines]).
- Voican CS, Martin S, Verstuyft C, Corruble E, Perlemuter G, Colle R. Liver function test abnormalities in depressed patients treated with antidepressants: a real-world systematic observational study in psychiatric settings. PLoS One. 2016;11:e0155234. [PMC free article: PMC4865191] [PubMed: 27171561](Among 321 psychiatric inpatients, only 116 [36%] had liver tests performed and only 18 during therapy with an antidepressant, 3 of which were suspected to have drug induced liver injury, 1 each with escitalopram, venlafaxine and amitriptyline, all without jaundice and 2 without symptoms, all 3 resolving).
- Friedrich ME, Akimova E, Huf W, Konstantinidis A, Papageorgiou K, Winkler D, Toto S, et al. Drug-induced liver injury during antidepressant treatment: results of AMSP, a drug surveillance program. Int J Neuropsychopharmacol. 2016;19(4):pyv126. pii. [PMC free article: PMC4851269] [PubMed: 26721950](Among 184,234 psychiatric inpatients from 80 hospitals, 149 cases [0.08%] of drug induced liver injury were reported including 71 of 50,201 patients treated with tricyclics, 18 of 11,876 receiving trimipramine and 2 of 2,016 receiving nortriptyline).
- Gahr M, Zeiss R, Lang D, Connemann BJ, Hiemke C, Schönfeldt-Lecuona C. Drug-Induced liver injury associated with antidepressive psychopharmacotherapy: an explorative assessment based on quantitative signal detection using different MedDRA terms. J Clin Pharmacol. 2016;56:769–78. [PubMed: 26470856](Using data on adverse drug reaction reports from the Uppsala Monitoring Center of WHO, there were higher relative hepatotoxicity reports for nefazodone, agomelatine, many tricyclics and mirtazapine).
- Chen VC, Lin CF, Hsieh YH, Liang HY, Huang KY, Chiu WC, Lee Y, McIntyre RS, et al. Hepatocellular carcinoma and antidepressants: a nationwide population-based study. Oncotarget. 2017;8:30464–70. [PMC free article: PMC5444756] [PubMed: 27783998](Among almost 50,000 cases of hepatocellular carcinoma registered in the Taiwan National Health Insurance Research Database, the rate of antidepressant use was lower than in approximately 250,000 matched controls from the database).
- Ferrajolo C, Scavone C, Donati M, Bortolami O, Stoppa G, Motola D, Vannacci A, et al. DILI-IT Study Group. Antidepressant-Induced Acute liver injury: a case-control study in an Italian inpatient population. Drug Saf. 2018;41:95–102. [PubMed: 28770534](Among 179 cases of hospitalizations for unexplained acute liver injury enrolled in an Italian prospective study between 2010 and 2014, 17 had been exposed to antidepressants including citalopram [n=4], sertraline [n=3], amitriptyline [n=3] and paroxetine [n=2], clomipramine [n=1] and amitriptyline [n=1]).
- Billioti de Gage S, Collin C, Le-Tri T, Pariente A, Bégaud B, Verdoux H, Dray-Spira R, et al. Antidepressants and hepatotoxicity: a cohort study among 5 million individuals registered in the French National Health Insurance Database. CNS Drugs. 2018;32:673–84. [PMC free article: PMC6061298] [PubMed: 29959758](Among 5 million persons identified in a national French health insurance database who started an antidepressant between 2010 and 2015, 382 developed serious liver injury resulting in hospitalization, rates per 100,0000 persons-years being 19 for SSRIs, 22 venlafaxine, 13 duloxetine, and 33 mirtazapine; conventional tricyclics and MAO inhibitors not discussed).
- Pladevall-Vila M, Pottegård A, Schink T, Reutfors J, Morros R, Poblador-Plou B, Timmer A, et al. Risk of acute liver injury in agomelatine and other antidepressant users in four European countries: a cohort and nested case-control study using automated health data sources. CNS Drugs. 2019;33:383–95. [PMC free article: PMC6441103] [PubMed: 30830574](Analysis of data sources from 4 European countries identified 3.2 million persons initiating antidepressant therapy among whom there was no increased risk for acute liver injury for agomelatine compared to citalopram, an SSRI with a low rate of hepatotoxicity).
- Drugs for depression. Med Lett Drugs Ther. 2020;62(1592):25–32. [PubMed: 32320387](Concise review of the mechanism of action, clinical efficacy, safety and costs of drugs for depression, mentions that tricyclics and MAO inhibitors remain valuable alternatives for treatment of moderate to severe depression, despite concerns about their safety; hepatotoxicity is mentioned only for nefazodone [now rarely used because of severe hepatotoxicity] and duloxetine [in heavy drinkers]).
- Ueberberg B, Frommberger U, Messer T, Zwanzger P, Kuhn J, Anghelescu I, Ackermann K, et al. Drug-induced liver injury (DILI) in patients with depression treated with antidepressants: a retrospective multicenter study. Pharmacopsychiatry. 2020;53:60–4. [PubMed: 31958850](Among 329 psychiatric inpatients with depression seen at 6 psychiatric centers in Germany, 17 [5%] had serum aminotransferase elevations but none had clinically apparent liver injury, most commonly implicated drugs included mirtazapine, agomelatine, citalopram and venlafaxine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Antidepressant Agents.[LiverTox: Clinical and Researc...]Review Antidepressant Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Safety of liver donation after fatal intoxication with the tricyclic antidepressant trimipramine.[Transplantation. 1996]Safety of liver donation after fatal intoxication with the tricyclic antidepressant trimipramine.Fattinger KE, Rentsch KM, Meier PJ, Dazzi H, Krähenbühl S. Transplantation. 1996 Nov 15; 62(9):1259-62.
- Controlled trial of trimipramine, monoamine oxidase inhibitors, and combined treatment in depressed outpatients.[Br Med J. 1979]Controlled trial of trimipramine, monoamine oxidase inhibitors, and combined treatment in depressed outpatients.Young JP, Lader MH, Hughes WC. Br Med J. 1979 Nov 24; 2(6201):1315-7.
- Review Trimipramine: a challenge to current concepts on antidepressives.[Eur Arch Psychiatry Clin Neuro...]Review Trimipramine: a challenge to current concepts on antidepressives.Berger M, Gastpar M. Eur Arch Psychiatry Clin Neurosci. 1996; 246(5):235-9.
- Shifts in metabolic parameters surrounding glucose homoeostasis resulting from tricyclic antidepressant therapy: implications of insulin resistance?[J Pharm Pharmacol. 2007]Shifts in metabolic parameters surrounding glucose homoeostasis resulting from tricyclic antidepressant therapy: implications of insulin resistance?Chadwick W, Wilson G, van de Venter M, Oelofsen W, Roux S. J Pharm Pharmacol. 2007 Jan; 59(1):95-103.
- Trimipramine - LiverToxTrimipramine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...