OVERVIEW
Introduction
Senna (Cassia species) is a popular herbal laxative that is available without prescription. Senna is generally safe and well tolerated, but can cause adverse events including clinically apparent liver injury when used in high doses for longer than recommended periods.
Background
Senna (sen’ a) belongs to a large genus of flowering plants found throughout the tropics, commonly used species being Cassia acutifolia (Alexandrian senna) and C. angustifolio (Indian or Tinnevelly senna). Extracts of the leaves, flowers and fruit of senna have been used for centuries in folk medicine as a laxative and stimulant. Senna is also included in several herbal teas, used for purging and in weight loss. The active components in senna extracts are anthraquinone derivatives and their glucosides, referred to as senna glycosides or sennosides. The sennosides are not absorbed but are hydrolyzed by colonic bacteria releasing the active moieties, rhein and rhein-anthrone which appear to act as local irritants on the colon, promoting peristalsis and evacuation. Sennosides and their metabolites may also enhance intestinal fluid accumulation and increase the moisture content of stool by inhibiting electrolyte and water reabsorption and increasing chloride secretion from the colon. Senna is minimally absorbed. Senna is used in many over-the-counter laxatives in combination with other agents under trade names such as Ex-lax, Fletcher’s Castoria and Senokot. The typical dose is 15 to 30 mg of sennosides twice a day, but is recommended for short term use only (less than one week). Side effects include abdominal cramps and electrolyte imbalance. Long term use or abuse can lead to “cathartic” colon with diarrhea, cramps, weight loss and darkened pigmentation of the colonic mucosa.
Hepatotoxicity
Use of senna in the recommended doses for a limited period of time has been associated with few side effects, most of which are mild and transient and related to its laxative action. With longer term and higher dose use of senna, however, adverse events have been described including several cases of clinically apparent liver injury. The time to onset of liver injury was usually after 3 to 5 months of use, and the pattern of serum enzyme elevations was hepatocellular. The liver injury was usually mild-to-moderate in severity and resolved rapidly with discontinuation. In at least one instance, reexposure led to rapid recurrence of liver injury. Immunoallergic features and autoimmune markers were not present in the published cases.
In addition, a related plant commonly known as coffee senna or Cassia orientalis has been linked to many instances of acute, severe toxicity with encephalopathy, myopathy and hepatic dysfunction. Outbreaks of “hepato-myo-encephopathy” of unknown cause among children occurred yearly in Uttar Pradesh, India typically between September and November. Investigation eventually identified Cassia occidentalis ingestion as the probable cause, typically occurring in children who eat the leaves or pods of the common flowering weed. While Cassia occidentalis has also been used to prepare tea, the amount ingested was minimal. In children, and rarely in adults, the presentation was precipitous with nausea, vomiting, tremor, abnormal and violent behavior, grimacing and self-mutilation followed by stupor and coma at which time serum aminotransferase and bilirubin levels were typically elevated. In severe instances, the liver injury was progressive, serum ammonia and INR levels rose and patients developed coma, convulsions and status epilepticus that was unresponsive to therapy. Autopsies revealed hepatic necrosis and cholestasis. A similar pattern of symptoms and injury occurs in animals that consume Cassia occidentalis. Whether this syndrome has a similar pathogenesis to the rare instance of hepatic injury attributed to typical senna (Cassia acutifolia or angustifolio) that is used as a laxative is unknown.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The liver injury due to senna has been attributed to the anthraquinone derivatives present in the herbal extract, and most cases have been associated with taking excessive doses suggesting a direct hepatotoxic rather than idiosyncratic etiology. Other anthraquinones used to treat constipation have been implicated in causing liver injury with long term use, including cascarosides (cascara) and hydroxyanthraquinone.
Outcome and Management
Liver injury from long term senna use is rare, and most cases have been self-limited and rapidly reversible upon stopping the laxative. However, cases with a severe course with signs of acute liver failure have been described. There is no evidence of cross sensitivity to hepatic damage with other laxatives. Restarting senna after hepatotoxicity has been associated with recurrence of liver injury and should be avoided.
Drug Class: Herbal and Dietary Supplements
Other Drugs in the Subclass, Anthraquinones: Cascara
CASE REPORT
Case 1. Acute liver injury due to Senna [Cassia angustifolia].(1)
A 52 year old woman was developed anorexia and progressive weakness followed by confusion and jaundice, having used large amounts of tea prepared from dry senna products to treat chronic constipation for several years. On presentation she was confused, weak and mildly jaundiced. Blood testing revealed serum glucose of 40 mg/dL, bilirubin 6.2 mg/dL, ALT 9160 U/L, AST 6640 U/L, GGT 160 U/L and INR 5.7. She also had lactic acidosis and renal insufficiency. She was admitted to the intensive care unit and soon required ventilation. Her coagulopathy worsened and she developed polyuria and phosphate wasting. She received intensive support and after several days began to improve. She was discharged after a 3 week hospitalization and, when seen one month later, was asymptomatic and all laboratory tests had returned to normal. Tests for hepatitis A, B and C, for autoantibodies and for acetaminophen levels were not reported. The botanicals used for making tea were analyzed and found to represent Cassia angustifolio without metal or other contaminants.
Key Points
Laboratory Values
Comment
The sudden onset of hepatic failure, lactic acidosis and encephalopathy is characteristic of acute hepatic necrosis due to a direct toxin. In this instance, the patient had been taking an excessive about of senna over a prolonged period, and it was not clear what precipitated this acute decompensation. Possible explanations are that it was triggered by a coincidental infection or dehydration or a change in the formulation of the Senna product. The general features of this clinical case report resemble those of hepato-myo-encephalopathy described from India in children who consume the pods or fruit of Cassia occidentalis. Conventional senna used as a laxative is quite safe, but these reports suggest that an overdose can cause acute hepatic failure with early signs of encephalopathy.
Case 2. Acute liver injury due to coffee senna [Cassia occidentalis].(2)
A 75 year old woman developed nausea, vomiting, diarrhea and low grade fever after medicating herself for two weeks with leaves of Senna occidentalis. She was seen at a local hospital and found to have a mildly elevated white blood cell count (12,000) with 80% neutrophils but normal serum bilirubin, ALT and alkaline phosphatase (Table). She was sent home but continued to worsen, developing confusion and jaundice and was admitted to a tertiary referral center 2 days later with stupor, deep jaundice and anasarca. Blood testing showed a serum bilirubin of 12.9 mg/dL (direct 10.4), ALT 130 U/L, Alk P 43 U/L, INR 2.68 and ammonia 127 µmol/L. Ultrasound revealed a normal sized liver and spleen and mild ascites. A lumbar puncture was normal. Tests for hepatitis A, B, C and E were negative. She was intubated and given antibiotics and fresh frozen plasma. She continued to deteriorate and developed coma, seizures, status epilepticus and died 2 days later, 5 days after her initial presentation.
Key Points
Laboratory Values
Comment
The sudden onset of nausea, vomiting and diarrhea followed shortly after by confusion, stupor and jaundice is typical of the syndrome of “hepato-myo-encephalopathy” described from India among children who consumed the fruit or pods of the common local weed Cassia occidentalis (coffee senna). This case was unusual in that it occurred in an adult and was not due to acute consumption but to several weeks of intake of the botanical as pain relief from chronic knee osteoarthritis. The liver injury is typical of acute hepatic necrosis or hyperacute liver failure with early signs of hepatic failure even when the bilirubin is normal or only mildly elevated. Hyperammonemia and coagulopathy arise early. This syndrome also has prominent neurologic signs and deaths are often due to encephalopathy rather than hepatic failure. The syndrome is not always fatal and, in some cases, intensive medical support appears to tide patients over the acute toxic effects. Recovery is rapid and seemingly complete. At issue is whether this syndrome is similar or related to the cases of acute liver injury that have been attributed to other Senna species such as Cassia angustifolia or acutifolia, which are used to produce the laxative commonly referred to as Senna (see Case 1).
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Senna – Generic
DRUG CLASS
Herbal and Dietary Supplements
SUMMARY INFORMATION
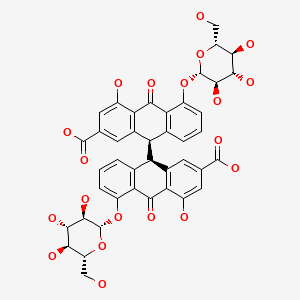
CHEMICAL FORMULA AND STRUCTURE
CITED REFERENCES
- 1.
- Beuers U, Spengler U, Pape GR. Hepatitis after chronic abuse of senna. Lancet. 1991;337:372–3. [PubMed: 1671276]
- 2.
- Ish P, Rathi S, Singh H, Anuradha S. Senna Occidentalis poisoning: an uncommon cause of liver failure. ACG Case Rep J. 2019;6:e00035. [PMC free article: PMC6658039] [PubMed: 31616727]
ANNOTATED BIBLIOGRAPHY
References updated: 01 April 2020
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 731-4.(Expert review of hepatotoxicity published in 1999; senna is not discussed).
- Seeff L, Stickel F, Navarro VJ. Hepatotoxicity of herbals and dietary supplements. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 631-58.(Review of hepatotoxicity of herbal and dietary supplements [HDS]; senna is not discussed).
- Senna. In, PDR for Herbal Medicines. 4th ed. Montvale, New Jersey: Thomson Healthcare Inc. 2007: pp. 743-7.(Compilation of short monographs on herbal medications and dietary supplements).
- Tolman KG, Hammar S, Sannella JJ. Possible hepatotoxicity of Doxidan. Ann Intern Med. 1976;84:290–2. [PubMed: 1259264](24 year old man developed fatigue 6 months after starting Doxidan [containing hydroxyanthraquinone for chronic constipation] and at 12 months developed nausea and syncope [bilirubin 0.8 mg/dL, AST 560 U/L, Alk P 339 U/L, ANA negative, slight neutropenia], liver biopsy showing chronic hepatitis, resolving within 1 month of stopping, recurring upon rechallenge).
- Beuers U, Spengler U, Pape GR. Hepatitis after chronic abuse of senna. Lancet. 1991;337:372–3. [PubMed: 1671276](26 year old nurse taking high doses of senna alkaloids developed jaundice and pruritus [bilirubin not given, ALT 303 U/L, Alk P 227 U/L], resolving within a few weeks of stopping and recurring upon restarting [ALT >280 U/L]).
- Franz G. The senna drug and its chemistry. Pharmacology. 1993;47 Suppl 1:2–6. [PubMed: 8234429](Senna is dried leaflets or fruits of Cassia senna, either acutifolia [Alexandrian] or angustifolia [Indian senna], native to Northern Africa, middle East and India, similar constituents including anthraquinone glycosides, called sennosides).
- Woolf GM. Senna-induced hepatotoxicity. Hepatology. 1999;550A:1560.(48 year old man developed jaundice 3 days after starting cascara sagrada [bilirubin 11.8 mg/dL, ALT 999 U/L, Alk P 309 U/L, ANA 1: 640] developing ascites, but recovering in 3 months).
- Nadir A, Reddy D, Van Thiel DH. Cascara sagrada-induced intrahepatic cholestasis causing portal hypertension: case report and review of herbal hepatotoxicity. Am J Gastroenterol. 2000;95:3634–7. [PubMed: 11151906](48 year old man developed jaundice 3 days after starting cascara sagrada [bilirubin 11.8 mg/dL, ALT 999 U/L, Alk P 309 U/L, ANA 1: 640], developing ascites, but recovering within 3 months of stopping).
- Wurtz AS, Vial T, Isoard B, Saillard E. Possible hepatotoxicity from Copaltra, an herbal medicine. Ann Pharmacother. 2002;36:941–2. [PubMed: 12014355](49 year old woman developed jaundice 3 months after starting Copaltra tea [Coutarea latiflora and Centaurium erythreae] for diabetes [bilirubin 20.8 mg/dL, ALT 3010 U/L, Alk P 132 U/L], with recovery in 3 months).
- De Smet PAGM. Herbal remedies. N Engl J Med. 2002;347:2046–56. [PubMed: 12490687](Review of status and difficulties of herbal medications, including lack of standardization, federal regulation, contamination, safety, hepatotoxicity and drug-herb interactions; specific discussion of 4 herbs with therapeutic promise: ginkgo, hawthorn, saw palmetto and St. John’s wort).
- Stedman C. Herbal hepatotoxicity. Semin Liver Dis. 2002;22:195–206. [PubMed: 12016550](Review and description of patterns of liver injury due to herbs, including discussion of potential risk factors, and herb-drug interactions; senna is listed as causing liver injury).
- Schiano TD. Hepatotoxicity and complementary and alternative medicines. Clin Liver Dis. 2003;7:453–73. [PubMed: 12879994](Comprehensive review of herbal associated hepatotoxicity; senna is not listed as causing hepatotoxicity).
- Vashishtha VM. Brief profile of an epidemic of acute encephalopathy in Western Uttar Pradesh. Indian Pediatr. 2003;40:920–2. [PubMed: 14530570](Letter describing the course of 19 children living in Western Uttar Pradesh in India who had the sudden onset of fever and vomiting followed by abnormal behavior, agitation, stupor and coma with rise in serum ALT and AST and coagulopathy, but modest bilirubin elevations and normal cerebral spinal fluid examination and no evidence of measles or other acute exanthem; 16 [87%] died).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, including 7 [5%] for herbal medications, none attributed to senna).
- Ernst E. Risks of herbal medicinal products. Pharmacoepidemiol Drug Saf. 2004;13:767–71. [PubMed: 15386721](Review of the adverse effects of over-the-counter herbal medications, focusing on the hepatotoxicity of kava, drug interactions with St. John’s wort, and contamination of traditional Chinese medications with heavy metals [arsenic, lead, mercury, thallium] and conventional western medications).
- Seybold U, Landauer N, Hillebrand S, Goebel FD. Senna-induced hepatitis in a poor metabolizer. Ann Intern Med. 2004;141:650–1. [PubMed: 15492352](28 year old woman developed jaundice after drinking herbal tea containing senna leaves, with positive rechallenge [ALT ~1390 U/L, GGT ~190 U/L], with Rhein anthrone detectable in serum).
- Vanderperren B, Rizzo M, Angenot L, Haufroid V, Jadoul M, Hantson P. Acute liver failure with renal impairment related to the abuse of senna anthraquinone glycosides. Ann Pharmacother. 2005;39:1353–7. [PubMed: 15956233](52 year old woman developed jaundice, acute liver failure, lactic acidosis, and phosphate loosing nephropathy after ingesting herbal tea made from senna fruits for 3 years [bilirubin 6.2 mg/dL, ALT 9160 U/L, INR 5.3], requiring temporary ventilator support, ultimately resolving [Case 1]).
- Sonmez A, Yilmaz MI, Mas R, Ozcan A, Celasun B, Dogru T, Taslipinar A, Kocar IH. Subacute cholestatic hepatitis likely related to the use of senna for chronic constipation. Acta Gastroenterol Belg. 2005;68:385–7. [PubMed: 16268429](77 year old male developed jaundice after taking a senna preparation for constipation for 3 months [bilirubin 4.9 rising to 16.9 mg/dL, ALT 657 U/L, Alk P 160 U/L], resolving within 1 month of stopping the herbal).
- Vashishtha VM, Nayak NC, John TJ, Kumar A. Recurrent annual outbreaks of a hepato-myo-encephalopathy syndrome in children in western Uttar Pradesh, India. Indian J Med Res. 2007;125:523–33. [PubMed: 17598938](Characterization of clinical course of hepatomyoencephalopathy occurring in outbreaks among children in Uttar Pradesh, India marked by sudden onset of fever, vomiting, abnormal behavior followed by stupor, coma and death [in 76%] within 72 hours, typically with marked ALT and AST elevations, hypoglycemia, abnormal coagulation studies but modest bilirubin elevations; liver biopsies showing acute hepatotoxic injury, central necrosis and hydropic changes, muscle biopsies showing focal necrosis and brain histology showing mild spongiosis and focal gliosis).
- Vashishtha VM, Kumar A, John TJ, Nayak NC. Cassia occidentalis poisoning causes fatal coma in children in western Uttar Pradesh. Indian Pediatr. 2007;44:522–5. [PubMed: 17684305](Home visits and family interviews of 10 Indian children with acute, severe hepatomyoencephalopathy found evidence that all 10 had consumed multiple Cassia occidentalis beans growing as weeds in their neighborhood shortly before becoming ill with liver injury, with stupor followed by coma and death [ALT 948-9160 U/L, CPK 42-4890 U/L, glucose <40 mg/dL in 6 of 10, prothrombin times abnormal in all]).
- Bruguera M, Herrera S, Lazaro E, Madurga M, Navarro M, De Abajo FJ. Gastroenterol Hepatol. 2007;30:66–8. [Acute hepatitis associated with consumption of Copalchi: a summary of 5 cases] [PubMed: 17335712](Five cases of hepatotoxicity of Copalchi in 4 men and 1 woman ages 59 to 77 years, taking herb for 2-13 months [bilirubin 0.7-13 mg/dL, ALT 403-865 U/L, GGT 54-116 U/L], resolving after stopping in all 5).
- García-Cortés M, Borraz Y, Lucena MI, Peláez G, Salmerón J, Diago M, Martínez-Sierra MC, et al. Liver injury induced by “natural remedies”: an analysis of cases submitted to the Spanish Liver Toxicity Registry. Rev Esp Enferm Dig. 2008;100:688–95. [PubMed: 19159172](Among 521 cases of drug induced liver injury submitted to Spanish registry, 13 [2%] were due to herbals, including one due to Cassia angustifolia [senna], with onset after 5 months [bilirubin 11.7 mg/dL, ALT 35 times ULN, Alk P 1.4 times ULN], resolving in one month).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 9% of cases were attributed to herbal medications, but none were linked to senna or cascara).
- Freeman HJ. "Melanosis" in the small and large intestine. World J Gastroenterol. 2008;14:4296–9. [PMC free article: PMC2731179] [PubMed: 18666316](Pigment found in small and large intestine with long term anthracene laxative [cascara, senna, aloes and rhubarb], use is usually lipofuscin rather than hemosiderin or melanin and is present in macrophages in the lamina propria).
- Soyuncu S, Cete Y, Nokay AE. Portal vein thrombosis related to Cassia angustifolia. Clin Toxicol (Phila). 2008;46:774–7. [PubMed: 19238740](42 year old woman developed abdominal pain and was found to have portal vein thrombosis 2 years after starting daily use of tea made from dried senna leaves [bilirubin normal, ALT 81 U/L]).
- Jacobsen C, Semb S, Kromann-Andersen H. Ugeskr Laeger. 2009;171:3367–9. [Toxic hepatitis following consumption of the herbal medicinal product Cascara Sagrada] Danish. [PubMed: 19925744](49 year old woman developed jaundice 4 weeks after starting daily ingestion of cascara sagrada for constipation [bilirubin 8.4 rising to 24.6 mg/dL, ALT 944 U/L, ascites], resolving within 4 months of stopping).
- Vitalone A, Menniti-Ippolito F, Raschetti R, Renda F, Tartaglia L, Mazzanti G. Surveillance of suspected adverse reactions to herbal products used as laxatives. Eur J Clin Pharmacol. 2012;68:231–8. [PubMed: 21964980](Among 519 adverse events attributed to herbals reported to an Italian registry between 2002 and 2011, 26 were related to herbal laxatives, including 3 cases of liver injury, 1 due to cascara and 2 to senna).
- Teschke R, Wolff A, Frenzel C, Schulze J, Eickhoff A. Herbal hepatotoxicity: a tabular compilation of reported cases. Liver Int. 2012;32:1543–56. [PubMed: 22928722](A systematic compilation of all publications on the hepatotoxicity of specific herbals identified 185 publications on 60 different herbs, including three reports implicating senna: Beuers [1991], Seybold [2004], Vanderperen [2005]).
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2013;37:3–17. [PubMed: 23121117](Systematic review of literature on HDS associated liver injury mentions that senna has been associated with at least 3 published cases of liver injury).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 15 [16%] due to herbal and dietary supplements, none of which were attributed to senna).
- Nirupam N, Sharma R, Chhapola V, Kanwal SK, Kumar V. Hepatomyoencephalopathy due to Cassia occidentalis poisoning. Indian J Pediatr. 2013;80:1063–4. [PubMed: 23255078](3 year old girl developed nausea, vomiting, excessive crying and self-mutilating behavior 3 days after eating Cassia orientalis beans growing in her neighborhood [bilirubin 1.9 mg/dL, ALT 3310 U/L, Alk P 1331 U/L, INR 2.7, ammonia 275 µg/L, CPK 1226 U/L], recovering with medical support after 8 days).
- Panigrahi G, Tiwari S, Ansari KM, Chaturvedi RK, Khanna VK, Chaudhari BP, Vashistha VM, et al. Association between children death and consumption of Cassia occidentalis seeds: clinical and experimental investigations. Food Chem Toxicol. 2014;67:236–48. [PubMed: 24614135](Injury similar to that in hepato-myo-encephopathy was produced in rodents using extracts of seeds of Cassia occidentalis with 2- to 3-fold increase in serum ALT levels, marked hypoglycemia and hepatic centrozonal necrosis).
- Teschke R, Genthner A, Wolff A, Frenzel C, Schulze J, Eickhoff A. Herbal hepatotoxicity: Analysis of cases with initially reported positive re-exposure tests. Dig Liver Dis. 2014;46:264–9. [PubMed: 24315480](Reanalysis of 34 published cases of liver injury due to herbal medications in which there was a reported positive rechallenge, finding only 21 [62%] fulfilled the criteria of a positive rechallenge using RUCAM, the others having inconsistent [18%] or incomplete data [21%]; among those with a confirmed positive rechallenge was one case due to senna [Beuers 1991]).
- Seeff LB, Bonkovsky HL, Navarro VJ, Wang G. Herbal products and the liver: a review of adverse effects and mechanisms. Gastroenterology. 2015;148:517–32.e3. [PubMed: 25500423](Extensive review of possible beneficial as well as harmful effects of herbal products on the liver does not discuss senna or cascara).
- Izzy M, Malieckal A, Little E, Anand S. Review of efficacy and safety of laxatives use in geriatrics. World J Gastrointest Pharmacol Ther. 2016;7:334–42. [PMC free article: PMC4848256] [PubMed: 27158549](Systematic review of 9 randomized controlled trials of laxatives in geriatric patients suggested that senna was the preferred agent in the elderly in regards to efficacy and safety; no mention of hepatotoxicity or restriction in the duration of therapy).
- García-Cortés M, Robles-Díaz M, Ortega-Alonso A, Medina-Caliz I, Andrade RJ. Hepatotoxicity by dietary supplements: A tabular listing and clinical characteristics. Int J Mol Sci. 2016;17:537. [PMC free article: PMC4848993] [PubMed: 27070596](Listing of published cases of liver injury from HDS products, but does not list or mention cases attributed to senna).
- Brown AC. Liver toxicity related to herbs and dietary supplements: Online table of case reports. Part 2 of 5 series. Food Chem Toxicol 2017; 107 (Pt A): 472-501. [PubMed: 27402097](Description of an online compendium of cases of liver toxicity attributed to HDS products, lists 4 reports of liver injury attributed to senna).
- Vega M, Verma M, Beswick D, Bey S, Hossack J, Merriman N, Shah A, et al. Drug Induced Liver Injury Network (DILIN). The incidence of drug- and herbal and dietary supplement-induced liver injury: preliminary findings from gastroenterologist-based surveillance in the population of the State of Delaware. Drug Saf. 2017;40:783–7. [PMC free article: PMC5699929] [PubMed: 28555362](A prospective, population based registry of cases of drug induced liver injury occurring in Delaware during 2014, identified 20 cases [2.7 per 100,000] overall, including 6 due to HDS products, all of which were proprietary multiingredient products, none specifically listing senna as a component).
- Alsalimy N, Madi L, Awaisu A. Efficacy and safety of laxatives for chronic constipation in long-term care settings: A systematic review. J Clin Pharm Ther. 2018;43:595–605. [PubMed: 29885259](Systematic review of 7 controlled trials of laxatives in 444 adults with chronic constipation found all agents equally effective and with no serious adverse events, common side effects being gastrointestinal discomfort, flatulence and diarrhea).
- Vilanova-Sanchez A, Gasior AC, Toocheck N, Weaver L, Wood RJ, Reck CA, Wagner A, et al. Are Senna based laxatives safe when used as long term treatment for constipation in children? J Pediatr Surg. 2018;53:722–7. [PubMed: 29429768](Among 640 children with chronic constipation treated with senna long term, 16 had side effects, most commonly abdominal pain and cramps or diarrhea, typically during the first few weeks of treatment, resolving spontaneously or with changing laxatives; 2% of children developed perineal rash or blisters, all with diarrhea and diaper use due to exposure to stool; no instances of liver injury reported).
- Chhapola V, Kanwal SK, Sharma AG, Kumar V. Hepatomyoencephalopathy secondary to Cassia occidentalis poisoning: report of three cases from North India. Indian J Crit Care Med. 2018;22:454–6. [PMC free article: PMC6020631] [PubMed: 29962748](Clinical course of 3 children with hepatomyoencephalopathy due to ingestion of Cassia Occidentalis seeds with fever, vomiting and stupor [bilirubin 2.8 to 5.1 mg/dL, ALT 1749 to 3310 U/L, Alk P 369 to 1331 U/L, INR 1.74 to 3.95], all receiving supportive care and surviving, but all 3 had a sibling who had recently died of a similar syndrome at home).
- Ish P, Rathi S, Singh H, Anuradha S. Senna Occidentalis poisoning: an uncommon cause of liver failure. ACG Case Rep J. 2019;6:e00035. [PMC free article: PMC6658039] [PubMed: 31616727](75 year old woman developed liver injury after 2 weeks of self-medication with Cassia occidentalis for osteoarthritis [bilirubin 0.8 rising to 18.7 mg/dL, ALT 42 rising to 542 U/L, Alk P 105 rising to 184 U/L, ammonia 127 µg/L, INR 1.28], dying of intractable status epilepticus and liver biopsy showing massive necrosis [Case 2]).
Publication Details
Publication History
Last Update: April 1, 2020.
Copyright
Publisher
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda (MD)
NLM Citation
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Senna. [Updated 2020 Apr 1].