By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001.

Immunobiology: The Immune System in Health and Disease. 5th edition.
Show detailsThe antigen receptors on B cells (the B-cell receptor or BCR) and T cells (the T-cell receptor or TCR) are multiprotein complexes made up of clonally variable antigen-binding chains—the heavy and light immunoglobulin chains in the B-cell receptor, and the TCRα and TCRβ chains in the T-cell receptor—that are associated with invariant accessory proteins. The invariant chains are required both for transport of the receptors to the cell surface and, most importantly, for initiating signaling when the receptors bind to an extracellular ligand. Antigen binding to the receptor generates signals that lead ultimately to the activation of nuclear transcription factors that turn on new gene expression and turn off genes typically expressed only in resting cells. In this part of the chapter we also see how clustering of the antigen receptors with co-receptors helps to generate these signals.
6-6. The variable chains of lymphocyte antigen receptors are associated with invariant accessory chains that carry out the signaling function of the receptor
The antigen-binding portion of the B-cell receptor complex is a cell-surface immunoglobulin that has the same antigen specificity as the secreted antibodies that the B cell will eventually produce. Indeed, it is identical to a secreted monomeric immunoglobulin, except that it is attached to the membrane through the carboxy termini of the paired heavy chains. The mRNA for the cell-surface heavy chain is spliced in such a way that the carboxy terminus of the protein is made up of a transmembrane domain and a very short cytoplasmic tail (see Fig. 4.19). The heavy and light chains do not by themselves make up a complete cell-surface receptor, however. When cells were transfected with heavy- and light-chain cDNA derived from a cell expressing surface immunoglobulin, the immunoglobulin that was synthesized remained inside the transfected cell rather than appearing on the surface. This implied that other molecules were required for the immunoglobulin receptor to be expressed on the cell surface. Two proteins associated with heavy chains on the B-cell surface were subsequently identified and called Igα and Igβ. Transfection of Igα and Igβ cDNA along with that for the immunoglobulin chains results in the appearance of a B-cell receptor on the cell surface.
One Igα chain and one Igβ chain associates with each surface immunoglobulin molecule. Thus the complete B-cell receptor is thought to be a complex of six chains—two identical light chains, two identical heavy chains, one Igα, and one Igβ (Fig. 6.7). The Igα and Igβ genes are closely linked in the genome and encode proteins composed of a single amino-terminal immunoglobulin-like domain connected via a transmembrane domain to a cytoplasmic tail. Igα and Igβ provide the only substantial cytoplasmic domains present in the receptor complex and are crucial for signaling. The transmembrane form of the immunoglobulin heavy chain has a very short cytoplasmic tail and it was hard to understand how this could signal into the cell that the surface immunoglobulin was ligated. The discovery of Igα and Igβ solved this intellectual problem.
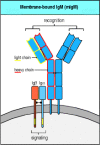
Figure 6.7
The B-cell receptor complex is made up of cell-surface immuno-globulin with one each of the invariant proteins Igα and Igβ. The immunoglobulin recognizes and binds antigen but cannot itself generate a signal. It is associated with antigen-nonspecific signaling (more...)
Signaling from the B-cell receptor complex depends on the presence in Igα and Igβ of amino acid sequences called immunoreceptor tyrosine-based activation motifs (ITAMs). These motifs were originally identified in the cytoplasmic tails of Igα and Igβ, but are now known to be present in the accessory chains involved in signaling from the T-cell receptor, and in the Fc receptors on mast cells, macrophages, monocytes, and natural killer (NK) cells that bind antibody constant regions. ITAMs are composed of two tyrosine residues separated by around 9–12 amino acids; the canonical ITAM sequence is ...YXX[L/V]X6–9YXX[L/V]..., where Y is tyrosine, L is leucine, V is valine, and X represents any amino acid. Igα and Igβ each have a single ITAM in their cytosolic tails, giving the B-cell receptor a total of two ITAMs. When antigen binds, the tyrosines in these ITAMs become phosphorylated by receptor-associated Src-family tyrosine kinases Blk, Fyn, or Lyn. The ITAMs, by virtue of their two precisely spaced tyrosines, are then able to bind with high affinity to the tandem SH2 domains of members of a second family of protein tyrosine kinases. As we will see in Section 6-9, these kinases—Syk in B cells and ZAP-70 in T cells—are important in transmitting the signal onward.
The complete α:β T-cell antigen receptor complex contains several different accessory chains in addition to the highly variable TCRα and TCRβ chains which form heterodimers containing a single antigen-binding site (see Chapter 4). The invariant accessory chains are CD3γ, CD3δ, and CD3ε, which make up the CD3 complex, and the ζ chain, which is present as a largely intracytoplasmic homodimer. Although the exact stoichiometry of the T-cell receptor complex is not definitively established, it seems likely that two α:β heterodimers are associated at the cell surface with one CD3γ, one CD3δ, two CD3ε, and one ζ homodimer (Fig. 6.8, one α:β heterodimer omitted). The three CD3 proteins are encoded in adjacent genes that are regulated as a unit and are required for surface expression of the α:β heterodimers and for signaling via the receptor. Optimal expression and maximum signaling, however, also require the ζ chain, which is encoded elsewhere in the genome.
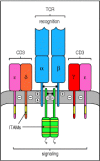
Figure 6.8
The T-cell receptor complex is made up of antigen-recognition proteins and invariant signaling proteins. The T-cell receptor α:β heterodimer (TCR) recognizes and binds its peptide:MHC ligand, but cannot signal to the cell that antigen (more...)
The CD3 proteins resemble Igα and Igβ in having an extracellular immunoglobulin-like domain and a single ITAM in their cytoplasmic tails. The ζ chain is distinct in having only a short extracellular domain, but it has three ITAMs in its cytoplasmic domain. The CD3 chains have negatively charged acidic residues in their transmembrane domains, which are able to interact with the positive charges of the α and β chains, as shown in Fig. 6.8. In total, the T-cell receptor complex is equipped with 10 ITAMs, which might give it greater flexibility in signaling compared with the B-cell receptor, as discussed later in this chapter.
Thus the antigen receptors of B and T lymphocytes are made from distinct sets of proteins but are similarly constructed. Both are molecular complexes made up of two types of functional component: variable chains that recognize the individual antigens and invariant chains that have a role both in the surface expression of the receptors and in transmitting signals to the cell's interior, enabling antigen recognition to be translated into action.
6-7. The ITAMs associated with the B-cell and T-cell receptors are phosphorylated by protein tyrosine kinases of the Src family
Phosphorylation of the tyrosines in ITAMs serves as the first intracellular signal indicating that the lymphocyte has detected its specific antigen. In B cells, three protein tyrosine kinases of the Src family—Fyn, Blk, and Lyn—are thought to be responsible for this. These Src-family kinases can associate with resting receptors through a low-affinity interaction with an unphosphorylated ITAM, to which they bind through a site in their amino-terminal domain. When the receptors cluster after antigen binding, the receptor-associated kinases phosphorylate and activate each other and are then thought to phosphorylate the tyrosine residues in the ITAMs of the cytoplasmic tails of Igβ and Igα. Phosphorylation of a single tyrosine in an ITAM allows the binding of a Src-family kinase through its SH2 domains, and kinases bound in this way are in turn activated to phosphorylate further ITAM tyrosine residues (Fig. 6.9).
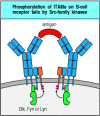
Figure 6.9
Src-family kinases are associated with antigen receptors and phosphorylate the tyrosines in ITAMs. The membrane-bound Src-family kinases Fyn, Blk, and Lyn associate with the B-cell antigen receptor by binding to ITAM motifs, either through their amino-terminal (more...)
The initial events in T-cell receptor signaling are also implemented by two Src-family kinases—Lck, which is constitutively associated with the cytoplasmic domain of the co-receptor molecules CD4 and CD8 (see Chapter 3), and Fyn, which associates with the cytoplasmic domains of the ζ and CD3ε chains upon receptor clustering. In the next section we discuss how CD4 or CD8 is clustered together with the antigen receptor when the receptor binds to its peptide:MHC ligand. Thus antigen recognition allows both Fyn and Lck to phosphorylate specific ITAMs on the accessory chains of the T-cell receptor complex.
The enzyme activity of the Src-family kinases is itself regulated by the phosphorylation status of the kinase domain and the carboxy-terminal region, each of which has regulatory tyrosine residues. Phosphorylation of the tyrosine in the kinase domain is activatory, while phosphorylation of a tyrosine at the carboxy terminus is inhibitory. Even after being phosphorylated at the activating tyrosine, Src-family kinases can be kept inactive by a protein tyrosine kinase called Csk (C-terminal Src kinase), which phosphorylates the inhibitory tyrosine (Fig. 6.10). As Csk activity is constitutive in resting cells, the Src proteins are generally inactive. An agent that counteracts the effects of Csk is the transmembrane protein tyrosine phosphatase CD45, also known as leukocyte common antigen, which is required for receptor signaling in lymphocytes and other cells. CD45 can remove the phosphate from phosphotyrosines, especially from the inhibitory tyrosine residue of Src-family kinases. Thus, the balance between Csk, which prevents the activation of Src-family kinases, and CD45, which restores their potential for being activated, determines the signaling activity of Src-family kinases in response to receptor aggregation. This is one way in which the threshold for initiating receptor signaling is regulated in lymphocytes. For example, as we will see in Chapter 10, activated effector T lymphocytes and memory T lymphocytes express an isoform of CD45 that associates with the T-cell receptor complex, thus facilitating signaling through the receptor. By contrast, naive T cells express a CD45 isoform that does not have this property.
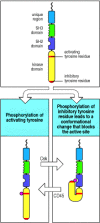
Figure 6.10
Regulation of Src-family kinase activity. Src-family kinases contain two tyrosine residues (red bars) that are targets for phosphorylation. Phosphorylation of the tyrosine in the kinase domain (bottom left panel) stimulates kinase activity, and this tyrosine (more...)
A second method by which the activity of Src-family kinases is regulated is by controlling the level at which they are present in the cell. Src-family kinases can be covalently modified with ubiquitin, a signal that targets proteins for degradation by the proteasome, and this degradation pathway is controlled through association with a regulatory protein, Cbl. Cbl itself does not appear to add the ubiquitin to the Src-family kinases; rather, it acts as an adaptor between the kinases and ubiquitin ligase enzymes. This process may be used to set a maximum level of response by limiting the concentration of kinases within the cell. However, Cbl is itself a target of tyrosine phosphorylation after receptor aggregation, and it seems more likely that its role is to switch off cascades of activated Src-family kinases after the cell has become activated.
In signaling from the antigen receptor, the activation of Src-family kinases is the first step in a signaling process that passes the signal on to many different molecules. Before we consider how the signals generated by Src-family kinases are transmitted onward, we will look at how antigen binding by both B cells and T cells directly or indirectly activates co-receptor molecules that are essential for producing a strong and effective intracellular signal.
6-8. Antigen receptor signaling is enhanced by co-receptors that bind the same ligand
Optimal signaling through the T-cell receptor complex occurs only when it clusters with the co-receptors CD4 or CD8. This is why most T cells with MHC class II-restricted T-cell receptors express CD4, which binds to the membrane-proximal domain of MHC class II molecules, whereas most T cells with MHC class I-restricted T-cell receptors express CD8, which binds MHC class I molecules. Expression of the appropriate co-receptor means that the peptide:MHC complex bound by the antigen receptor can be simultaneously bound by a co-receptor molecule. About 100 identical specific peptide:MHC complexes are required on a target cell to trigger a T cell expressing the appropriate co-receptor. In the absence of the co-receptor, 10,000 identical complexes (about 10% of all the MHC molecules on a cell) are required for optimal T-cell activation. This density is rarely, if ever, achieved in vivo.
Aggregation of the T-cell receptor with the appropriate co-receptor helps to activate the T cell by bringing the Lck tyrosine kinase associated with the cytoplasmic domain of the co-receptor together with the ITAMs and other targets associated with the cytoplasmic domains of the T-cell receptor complex (Fig. 6.11). One of the principal targets of Lck in T cells is another kinase, the ζ-chain-associated protein or ZAP-70, which is important in propagating the signal onward, as we will see in the next section.
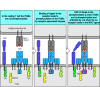
Figure 6.11
Clustering of the T-cell receptor and a co-receptor initiates signaling within the T cell. When T-cell receptors become clustered on binding MHC:peptide complexes on the surface of an antigen-presenting cell, activation of receptor-associated kinases (more...)
B-cell receptor signaling is also enhanced by aggregation with a co-receptor. The B-cell co-receptor is expressed on mature B cells as a complex of the cell-surface molecules CD19, CD21, and CD81. One way in which this complex can be co-ligated with the B-cell receptor is through the recognition of an antigen that has activated complement. The CD21 molecule (also known as complement receptor 2, CR2) is a receptor for the C3d fragment of complement, so antigens, such as pathogens, that have activated complement directly (see Chapter 2) or through the activation of antibody can cross-link the B-cell receptor with CD21 and its associated proteins. This induces phosphorylation of the cytoplasmic tail of CD19 by B-cell receptor-associated tyrosine kinases, which in turn leads to the binding of Src-family kinases and the recruitment of a lipid kinase called phosphatidylinositol 3-OH kinase (PI 3-kinase) (Fig. 6.12). Co-ligation of the B-cell receptor with its CD19/CD21/CD81 co-receptor increases signaling 1000- to 10,000-fold. The role of the third component of the B-cell receptor complex, CD81 (TAPA-1), is as yet unknown.
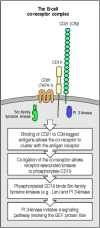
Figure 6.12
B-cell antigen receptor signaling is modulated by a co-receptor complex of at least three cell-surface molecules, CD19, CD21, and CD81. Binding of the cleaved complement fragment C3d to antigen allows the tagged antigen to bind to both the B-cell receptor (more...)
CD19 is expressed on all B cells from an early stage in their development, before CD21 and CD81 are expressed, and it appears to contribute to signaling through the B-cell receptor even in the absence of co-ligation through CD21. Thus B-cell activation that is experimentally induced by cross-linking the B-cell receptor with anti-receptor antibodies is accompanied by co-capping of CD19, the tyrosine phosphorylation of its cytoplasmic tail, and the activation of PI 3-kinase. By contrast, B cells from mice that lack CD19 fail to proliferate in response to B-cell receptor cross-linking and do not fully activate the intracellular signaling pathways normally generated when the B-cell receptor is cross-linked. These experiments suggest that CD19 can associate with the B-cell receptor, either constitutively or after receptor activation, and contribute to signaling even when the co-receptor has not been engaged through CD21 binding to complement. The physiological importance of CD19 is demonstrated by genetically engineered mice that lack this molecule; these mice make deficient B-cell responses to most antigens.
6-9. Fully phosphorylated ITAMs bind the protein tyrosine kinases Syk and ZAP-70 and enable them to be activated
Once the ITAMs in the receptor cytoplasmic tails have been phosphorylated, they can recruit the next players in the signaling cascade, the protein tyrosine kinases Syk in B cells and ZAP-70 (ζ-chain-associated protein) in T cells. These two proteins define a second family of protein tyrosine kinases expressed mainly in hematopoietic cells (Syk) or mainly in T lymphocytes (ZAP-70); they have two SH2 domains in their amino-terminal halves and a carboxy-terminal kinase domain. As each SH2 domain binds to one phosphotyrosine, these proteins preferentially bind to motifs with two phosphotyrosines spaced a precise distance apart; tyrosines spaced correctly are found in the ITAM motif. Thus, ZAP-70 or Syk molecules are recruited to the receptor complex upon full phosphorylation of the ITAMs.
Until Syk has bound to the doubly phosphorylated ITAM in the B-cell receptor, it is inactive enzymatically. To become active it must itself be phosphorylated, and this is thought to occur by transphosphorylation mediated by Syk itself or by Src kinases. Each B-cell receptor complex contains two molecules of Syk, bound to the Igα and Igβ chains. Once the receptors are clustered, these receptor-associated kinases are brought into contact with each other and are thus able to phosphorylate, and hence activate, each other (Fig. 6.13). Once activated, Syk phosphorylates target proteins to initiate a cascade of intracellular signaling molecules, which will be described in the next section.
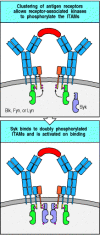
Figure 6.13
Full phosphorylation of the ITAMs on clustered Igα or β chains associated with the B-cell receptor creates binding sites for Syk and Syk activation via transphosphorylation. On clustering of the receptors, the receptor-associated tyrosine kinases (more...)
ZAP-70 is not activated by transphosphorylation after binding to the ITAMs in the ζ chain; instead, it is activated by the co-receptor-associated Src kinase Lck (see Fig. 6.11). Once activated, ZAP-70 phosphorylates the substrate LAT (linker of activation in T cells) and the protein called SLP-76, a second linker or adaptor protein in T cells. LAT is a cytoplasmic protein containing multiple tyrosines and associates with the inside face of the plasma membrane through cysteine residues that are palmitoylated and thus become associated with membrane lipid rafts. The association of LAT with lipid rafts, small, cholesterol-rich areas in the membrane, is essential for recruitment of a signaling complex and the propagation of signals away from the membrane and on into the interior of the cell. By virtue of its many tyrosine residues, phosphorylated LAT is able to recruit proteins that bind through their SH2 domains and transmit the signal from the T-cell membrane to downstream targets. A protein serving the same function in B cells has recently been identified as SLP-65 or BLNK for B-cell linker protein. This, again, has multiple sites for tyrosine phosphorylation, and interacts with many of the same proteins as LAT and SLP-76, to which it has strong homology in sequence.
6-10. Downstream events are mediated by proteins that associate with the phosphorylated tyrosines and bind to and activate other proteins
Once ZAP-70 tyrosine kinase has been activated in T cells, and LAT and SLP-76
have been phosphorylated, the next steps in the signaling pathway serve to
propagate the signal at the cell membrane, and eventually to communicate it to
the nucleus. A very similar signaling pathway exists in B cells, made up of
activated Syk acting on BLNK, and again propagating the signal from the cell
membrane into the nucleus. Signal propagation involves various proteins that
bind to phosphotyrosine via SH2 domains, and proteins that are targets for
protein tyrosine kinases. Several classes of protein participate in signal
propagation. These include the enzyme PLC-γ (see Section 6-4), which initiates two of the main signaling pathways
that lead to the nucleus. The third main pathway is generated by activation of
the small G protein Ras. These three pathways propagate the signal from the
activated receptors at the plasma membrane and carry it to the nucleus, as
illustrated in Fig. 6.14 for B cells and
Fig. 6.15 for T cells. We will
discuss in more detail below how each of these pathways is initiated and, in
Section 6-11, how they lead to
changes in gene expression in the nucleus. Activation of PLC-γ is linked to
activation of the antigen receptor through a family of Src-like tyrosine kinases
called Tec kinases, after a member
of the family which is expressed in T and B cells. Other Tec kinases with an
important role in lymphocytes are Btk, which is expressed in B lymphocytes and
Itk, which is expressed in T lymphocytes. These kinases contain a domain, called
a pleckstrin-homology (PH) domain, that allows them to interact with
phosphorylated lipids on the inner face of the cell membrane. They also carry
SH2 and SH3 domains that allow them to interact with the adaptor proteins LAT,
SLP-76, and BLNK. These interactions allow Tec kinases to cluster around the
activated antigen receptor where they are phosphorylated and activated by other
Src-family tyrosine kinases. Once activated, Tec kinases phosphorylate and
activate PLC-γ, which cleaves inositol phospholipids to generate the
intracellular second messengers diacylglycerol and IP3 (see Section 6-4). The importance of the Tec
kinases in lymphocytes can be seen from inherited deficiencies in these enzymes.
The human immunodeficiency disease X-linked agammaglobulinemia (XLA) ( X-linked Agammaglobulinemia, in
Case Studies in Immunology, see Preface for details), in which B
cells fail to mature (see Section 11-8),
results from a mutation of Btk, while the same gene mutated in mice leads to a
similar immunodeficiency called xid.
X-linked Agammaglobulinemia, in
Case Studies in Immunology, see Preface for details), in which B
cells fail to mature (see Section 11-8),
results from a mutation of Btk, while the same gene mutated in mice leads to a
similar immunodeficiency called xid.
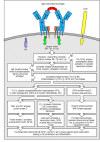
Figure 6.14
Simplified outline of the intracellular signaling pathways initiated by cross-linking of B-cell receptors by antigen. Cross-linking of surface immunoglobulin molecules activates the receptor-associated Src-family protein tyrosine kinases Blk, Fyn, and (more...)
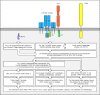
Figure 6.15
Simplified outline of the intracellular signaling pathways initiated by the T-cell receptor complex and its co-receptor. The T-cell receptor complex and co-receptor (in this example the CD4 molecule) are associated with Src-family protein kinases, Fyn (more...)
The activation of Ras is achieved by adaptor proteins and guanine-nucleotide exchange factors (see Section 6-5) recruited to the phosphorylated receptors. In B lymphocytes, the adaptor protein Shc binds to tyrosine residues that have been phosphorylated by the receptor-associated tyrosine kinases. Another adaptor protein, Grb2, which has an SH2 domain flanked on both sides by SH3 domains, forms a complex with Shc and this complex binds the guanine-nucleotide exchange factor SOS. SOS, in turn, is involved in activating Ras by the mechanism shown in Fig. 6.6. In T lymphocytes, the adaptor protein GADS, a homologue of Grb2, is recruited by phosphorylated LAT, which again uses SOS to recruit Ras to the pathway. Adaptor proteins thus form the scaffolding of a signaling complex, associated with lipid rafts, that links ligand binding by the antigen receptor at the cell surface to the activation of Ras, which then triggers further signaling events downstream.
Another small G protein is activated via the B-cell co-receptor complex (see Section 6-8). Phosphorylated CD19 binds a multifunctional intracellular signaling molecule called Vav; this is an adaptor protein that also contains guanine-nucleotide exchange factor activity. When Vav is activated, it can activate the small G protein Rac. Small G proteins such as Ras and Rac activate a cascade of protein kinases that leads directly to the phosphorylation and activation of transcription factors; we will discuss this in the next section. Vav and Rac can also influence changes in the actin cytoskeleton.
Thus, as we have seen so far, a signal originating from activated tyrosine kinases at the cell membrane can be propagated through several different pathways involving many intracellular proteins. We now turn to the question of how signals are transmitted to the nucleus, there to activate transcription factors that can regulate specific genes.
6-11. Antigen recognition leads ultimately to the induction of new gene synthesis by activating transcription factors
The ultimate response of lymphocytes to extracellular signals is the induction of new gene expression. This is achieved through the activation of transcription factors—proteins that control the initiation of transcription by binding to regulatory sites in the DNA. Several important transcription factors involved in lymphocyte responses to antigen are activated as a consequence of phosphorylation by the MAP kinases (see Section 6-5). These kinases are themselves activated by phosphorylation; in the inactive, nonphosphorylated state they are resident in the cytoplasm, but when activated by phosphorylation they translocate into the nucleus.
Transcription factors are activated by MAP kinases by the general pathway shown in Fig. 6.16. In lymphocytes, the MAP kinases that are thought to activate transcription factors in response to antigen-receptor ligation are called Erk1 (for extracellular-regulated kinase-1) and Erk2. MAP kinases are unusual, in that their full activation requires phosphorylation on both a tyrosine and a threonine residue, which are separated in the protein by a single amino acid. This can be done only by MAP kinase kinases, enzymes with dual specificity for tyrosine and serine/threonine residues. In the context of antigen-receptor signaling, the MAP kinase kinases that activate Erk1 and Erk2 are called Mek1 and Mek2. MAP kinase kinases are themselves activated by phosphorylation by a MAP kinase kinase kinase, which is the first kinase in the cascade. It is thought that in lymphocytes this MAP kinase kinase kinase is the serine/threonine kinase Raf (Fig. 6.17, left panel). The antigen receptor activates this cascade through the activation of Raf by the GTP-bound form of Ras.

Figure 6.16
MAP kinase cascades activate transcription factors. All MAP kinase cascades share the same general features. They are initiated by a small G protein, which is switched from an inactive state to an active state by a guanine-nucleotide exchange factor (GEF). (more...)
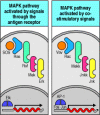
Figure 6.17
Initiation of MAP kinase cascades by guanine-nucleotide exchange factors is involved in both antigen receptor and co-stimulatory signaling. Signaling through the antigen receptors of both B and T cells (left panel) leads to the activation of the guanine-nucleotide (more...)
The activation of transcription factors through a MAP kinase cascade is a critical part of many cell signaling pathways. How the signal is individualized for different stimuli is not yet known, although the existence of variant components at each level of the pathway may allow this to occur. For example, the MAP kinase cascade triggered by signals from the B-cell co-receptor through Vav and Rac uses different kinases from those in the antigenreceptor pathway, and activates different transcription factors (Fig. 6.17, right panel). The MAP kinase cascade activated by the antigen receptor activates the transcription factor Elk, which in turn upregulates the synthesis of the transcription factor Fos. By contrast, the MAP kinase pathway activated by co-ligation of the B-cell co-receptor activates the transcription factor Jun. These pathways can combine in their effects because heterodimers of Fos and Jun form AP-1 transcription factors, which regulate the expression of many genes involved in cell growth. It appears that both pathways are required to drive the clonal expansion of naive lymphocytes. In T cells, the MAP kinase pathway that activates Jun is activated through a cell-surface molecule known as CD28, which interacts with co-stimulatory molecules that are induced on the surface of antigen-presenting cells during the innate immune response to infection (see Section 2-18). Thus this second MAP kinase pathway appears to convey to the cell that the antigen is part of a pathogen that has been recognized by the innate immune system.
A transcription factor that is activated through antigen-receptor signaling, but by a different pathway, is NFAT (nuclear factor of activated T cells). This is something of a misnomer, as NFAT transcription factors are found not only in T cells but also in B cells, NK cells, mast cells, and monocytes, as well as in some nonhematopoietic cells. The function of NFAT is regulated by its intracellular location. NFAT contains amino acid sequence motifs specifying a nuclear localization signal, which enables it to be translocated into the nucleus, and a nuclear export signal, which directs the movement of NFAT back into the cytosol. In unstimulated cells, the nuclear localization signal is rendered inoperative by phosphorylation at serine/threonine residues; hence NFAT is retained in the cytosol after its synthesis. In addition, should any NFAT make its way to the nucleus, the nuclear export sequence also becomes phosphorylated by a kinase, glycogen synthase kinase 3 (GSK3), which resides primarily within the nucleus. The phosphorylated NFAT is then rapidly exported from the nucleus. The sum of these two effects is that all the NFAT in a resting cell is found in the cytosol.
NFAT is released from the cytosol by the action of the enzyme calcineurin, a serine/threonine protein phosphatase. Calcineurin is itself activated by the increase in intracellular free Ca2+ that accompanies lymphocyte activation (see Fig. 6.15). Once NFAT has been dephosphorylated by calcineurin, it enters the nucleus, where it can act as a transcriptional regulatory protein. However, activation of calcineurin alone is insufficient to allow NFAT to function within the nucleus. In the absence of other signals, the NFAT is phosphorylated by GSK3 and exported back out of the nucleus again. Other signals mediated through the T-cell receptor are required to downregulate the activity of GSK3 and permit NFAT to remain in the nucleus. These other signals, and signals generated through co-stimulation, may contribute to NFAT function in another way, as NFAT acts as a transcriptional regulator in combination with AP-1 transcription factors, which, as we have seen above, are dimers of Fos and Jun. In lymphocytes, NFAT interacts with Fos:Jun heterodimers that have been activated by the MAP kinase known as Jnk (or ‘Junk’, as it is commonly called) (see Fig. 6.17, right panel). This illustrates how complex the process of signal transduction can be, and how proteins that regulate transcription can integrate signals that come from different pathways.
The importance of NFAT in T-cell activation is illustrated by the effects of the
selective inhibitors of NFAT called cyclosporin A and FK506 (tacrolimus). These
drugs inhibit calcineurin and hence prevent the formation of active NFAT. T
cells express low levels of calcineurin, so they are more sensitive to
inhibition of this pathway than are many other cell types. Both cyclosporin A
and FK506 thus act as effective inhibitors of T-cell activation with only
limited side-effects (see Chapter
14). These drugs are used widely to prevent graft rejection, which
they do by inhibiting the activation of alloreactive T cells. ( Insulin-Dependent Diabetes Mellitus, in
Case Studies in Immunology, see Preface for details)
Insulin-Dependent Diabetes Mellitus, in
Case Studies in Immunology, see Preface for details)
6-12. Not all ligands for the T-cell receptor produce a similar response
So far, we have assumed that all peptide:MHC complexes that are recognized by a given T-cell receptor will activate the T cell equally—that is, that the T-cell receptor is a binary switch with only two settings, ‘on’ and ‘off.’ Peptides that trigger the ‘on’ setting are called agonist peptides, by analogy with the agonist drugs that activate other types of receptor. However, experiments originally designed to explore the structural basis for antigen recognition by T cells unexpectedly showed that recognition does not necessarily lead to activation. Indeed, some variant peptide:MHC complexes that do not themselves activate a given T cell can actually inhibit its response to the agonist peptide:MHC complex. These peptides are usually called antagonist peptides, as they antagonize the action of the agonist peptide. Other peptides that can trigger only a part of the program activated by the agonist peptide are called altered peptide ligands or partial agonists. Such ligands can, for instance, induce the lymphocyte to secrete cytokines but not to proliferate. Most of the antagonist and partial agonist peptides that have been described are closely related in sequence to the agonist peptide, although this is not always the case. Several studies have suggested that the binding interactions between T-cell receptors and these variant peptide:MHC complexes are of a lower affinity than the binding interactions between a T-cell receptor and its agonist peptide:MHC ligand.
The extent to which antagonist peptides and altered peptide ligands influence physiological immune responses is not known, although they might contribute to the persistence of some viral infections. For example, mutant peptides of epitopes on cells infected with human immunodeficiency virus (HIV) can inhibit the activation of CD8 T cells specific for the original agonist epitope at a 1:100 ratio of mutant to agonist; this sort of effect could allow cells infected with a mutant virus, which arise commonly during the course of HIV infection, to inhibit virus-specific cytotoxic T cells and thereby promote the survival of all infected cells.
Differential signaling by variants of agonist peptides is thought to be important for the development of T cells in the thymus and their subsequent maintenance in the periphery. As we will discuss in Chapter 7, T cells are selected for their potential to be activated by foreign peptide antigens in the context of self MHC molecules through interactions with self peptide:self MHC complexes. This process seems to require the receipt of survival signals from self peptide:self MHC complexes that are related to, but not identical with, the foreign peptide:self MHC complexes that the mature T cell will recognize. We will discuss the evidence for survival signaling by altered peptide ligands further in Section 6-20.
The basis of the incomplete activating signal delivered by altered peptide ligands is unknown, but it has been shown that the recognition of these ligands leads to altered phosphorylation of CD3ε and ζ chains, and to the recruitment of the inactive form of ZAP-70 tyrosine kinase to the T-cell receptor. Cross-linking the T-cell receptor alone, without any co-receptor engagement, can also generate the same partial phosphorylation events within the cell. Thus, the incomplete signal generated by altered peptide ligands might reflect a failure to recruit the co-receptors or a failure of the T-cell receptor to interact with the co-receptor productively. Also, because the affinity of the T-cell receptor for the altered peptide ligand:MHC complex is lower than that for the activating complex, the T-cell receptor might dissociate too quickly from its ligand for a full activating signal to be delivered. Another possibility is that conformational changes in the T-cell receptor can contribute to the life-span of the signaling complex and its ability to recruit Lck, and that altered peptide ligands fail to trigger these conformational changes. Antagonist signaling also recruits the tyrosine phosphatase SHP-1 to the T-cell receptor signaling complex, where it can dephosphorylate the signaling complex (see Section 6-14). This recruitment, which is seen late in activation by agonist peptides, occurs within one minute in T cells recognizing antagonist peptides.
6-13. Other receptors on leukocytes also use ITAMs to signal activation
Although this chapter is focused on lymphocyte antigen receptors, which are the signaling machines that regulate adaptive immunity, other receptors on immune system cells also use the ITAM motif to transduce activating signals (Fig. 6.18). One example is FcγRIII (CD16); this is a receptor for IgG that triggers antibody-dependent cell-mediated cytotoxicity (ADCC) by NK cells, which we will learn about in Chapter 9; it is also found on macrophages and neutrophils, where it facilitates the uptake and destruction of antibody-bound pathogens. FcγRIII is associated with either an ITAM-containing ζ chain like those found in the T-cell receptor complex or with a second member of the same protein family known as the γ chain. The γ chain is also associated with another type of receptor—the Fcε receptor I (FcεRI) on mast cells. As we will discuss in Chapter 12, this receptor binds IgE antibodies and on cross-linking by allergens it triggers the degranulation of mast cells.
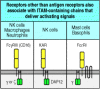
Figure 6.18
Other receptors that pair with ITAM-containing chains can deliver activating signals. Cells other than B and T cells have receptors that pair with accessory chains containing ITAMs, which are phosphorylated when the receptor is cross-linked. These receptors (more...)
Other members of the ITAM-containing ζ-chain family have recently been discovered in NK cells. These proteins, called DAP10 and DAP12, each have only one ITAM, but in other respects they are similar to ζ chains and can form ζ-like homodimers in the membranes of NK cells. Both DAP10 and DAP12 associate with receptors called killer activatory receptors (KARs). As we discussed in Section 2-27, these receptors can activate NK cells to kill infected or abnormal target cells. The killer activatory receptors signal through their associated ITAM-containing homodimer for the release of the cytotoxic granules by which NK cells kill their targets.
Several viral pathogens appear to have acquired ITAM-containing receptors from their hosts. These include the Epstein-Barr virus (EBV), whose LMP2A gene has a cytoplasmic tail which encodes an ITAM. This allows EBV to trigger B-cell proliferation by using the downstream signaling molecules we discussed in Sections 6-9 to 6-11. Other viruses also express genes that encode proteins with ITAMs in their transmembrane regions and also induce transformation. An example is the Kaposi sarcoma herpes virus (KSHV or HHV8).
6-14. Antigen-receptor signaling can be inhibited by receptors associated with ITIMs
Both B and T cells receive signals that can counteract and modify the activating signals delivered through antigen receptors and co-receptors. These inhibitory signals usually block the response by raising the threshold at which signal transduction can occur. Most of these modifying signals are received through receptors that bear a distinct motif called an immunoreceptor tyrosine-based inhibitory motif (ITIM) in their cytoplasmic tails. In this motif, a large hydrophobic residue such as isoleucine or valine occurs two residues upstream of a tyrosine that is followed by two amino acids and a leucine to give the amino acid sequence ...[I/V]XYXXL... (Fig. 6.19).
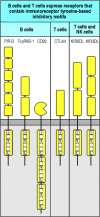
Figure 6.19
Some lymphocyte cell-surface receptors contain motifs involved in downregulating activation. Several receptors that transduce signals that inhibit lymphocyte or NK cell activation contain ITIMs (immunoreceptor tyrosine-based inhibitory motifs) in their (more...)
The ITIM motif is found in several receptors that modulate activation signals in lymphocytes. It functions by recruiting one or other of the inhibitory phosphatases SHP-1, SHP-2 and SHIP. These phosphatases carry one or more SH2 domains that preferentially bind the phosphorylated tyrosines in the ITIM. SHP-1 and SHP-2 are protein tyrosine phosphatases and remove the phosphate groups added by tyrosine kinases. SHIP is an inositol phosphatase and removes the 5′ phosphate from phosphatidylinositol trisphosphate (PI-3,4,5-P); the exact mechanism by which SHIP regulates signaling is not known, but it is thought to inhibit the activation of PLC-γ by inhibiting the recruitment of the Tec family of kinases, including Btk and Itk, and thus the production of DAG and IP3 and the associated mobilization of calcium.
One example of an ITIM-containing receptor that inhibits lymphocyte activation is the B-cell Fc receptor for IgG known as FcγRIIB-1. It has long been known that the activation of naive B cells in response to antigen can be inhibited by soluble IgG antibodies that recognize the same antigen and therefore co-ligate the B-cell receptor with this Fc receptor. It was only recently, however, that the FcγRIIB-1 ITIM motif was defined and shown to function by drawing SHIP into a complex with the B-cell receptor.
Several other receptors on B and T cells contain the ITIM motif and inhibit cell activation when they are ligated along with the antigen receptors. One example is CD22, a B-cell transmembrane protein that inhibits B-cell signaling. PIR-B, the paired immunoglobulin-like receptor, also expressed on B cells, contains an ITIM that allows it to interact with the tyrosine phosphatase SHP-1. In T cells, the transmembrane protein CTLA-4 is induced by activation and then has a critical role in regulating T-cell signaling; it binds to the same co-stimulatory molecules as CD28 and inhibits signaling through the T-cell receptor by recruiting the tyrosine phosphatase SHP-2. Antibodies that block recognition by CTLA-4 of its ligands lead to massive increases in T-cell effector function. We will return to the therapeutic consequences of this in Chapter 14, when we look at how T cells can be induced to reject established tumors. The killer inhibitory receptors (KIR) on NK cells and T cells also have ITIM motifs; they recognize MHC class I molecules and transmit signals that inhibit the release of cytotoxic granules when NK cells recognize healthy uninfected cells (see Section 2-27). These examples illustrate how the ITIM motif is able to modify signals originating from the B-cell receptor, the T-cell receptor, Fc receptors, and NK cell receptors, even to the extent of completely negating them.
Summary
Lymphocyte antigen receptors are multiprotein complexes made up of variable antigen-binding chains and invariant chains that transmit the signal that antigen has bound. The cytoplasmic tails of the invariant chains contain amino acid motifs called ITAMs, each possessing two tyrosine residues, that are targeted by receptor-associated protein tyrosine kinases of the Src family upon receptor aggregation. The B-cell receptor complex is associated with two such ITAMs, whereas the T-cell receptor complex is associated with 10, the larger number allowing the T-cell receptor a greater flexibility in signaling. Once the ITAMs have been phosphorylated by an Src-family kinase, the Syk-family kinases—Syk in B cells and ZAP-70 in T cells—bind and become activated. Linker and adaptor proteins are subsequently phosphorylated and serve to recruit enzymes that are activated by relocalization to the plasma membrane, by phosphorylation, or by both. Receptor phosphorylation initiates several signaling pathways, including those propagated through phospholipase C-γ and the small G proteins. These pathways converge on the nucleus and result in new patterns of gene expression. The small G proteins activate a cascade of serine/threonine protein kinases known as a MAP kinase cascade, which leads to the phosphorylation and activation of transcription factors. Signaling through the antigen receptors can be enhanced by signaling through the B-cell co-receptor on B cells or the CD4 and CD8 co-receptor molecules on T cells; signaling through the co-stimulatory receptor CD28 on T cells also contributes to activating naive T cells. Activating signals can be modulated or inhibited by signals from inhibitory receptors that are associated with chains containing a different motif, ITIM, in their cytoplasmic tails. This provides a mechanism for tuning the on/off threshold according to external stimuli or the state of development of the cell, allowing the modulation of the adaptive immune response. Signaling through the T-cell receptor also occurs in response to altered peptide or antagonist ligands, leading to a state of partial activation that can affect cell survival and the response to agonist ligands.
- The variable chains of lymphocyte antigen receptors are associated with invariant accessory chains that carry out the signaling function of the receptor
- The ITAMs associated with the B-cell and T-cell receptors are phosphorylated by protein tyrosine kinases of the Src family
- Antigen receptor signaling is enhanced by co-receptors that bind the same ligand
- Fully phosphorylated ITAMs bind the protein tyrosine kinases Syk and ZAP-70 and enable them to be activated
- Downstream events are mediated by proteins that associate with the phosphorylated tyrosines and bind to and activate other proteins
- Antigen recognition leads ultimately to the induction of new gene synthesis by activating transcription factors
- Not all ligands for the T-cell receptor produce a similar response
- Other receptors on leukocytes also use ITAMs to signal activation
- Antigen-receptor signaling can be inhibited by receptors associated with ITIMs
- Summary
- Antigen receptor structure and signaling pathways - ImmunobiologyAntigen receptor structure and signaling pathways - Immunobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...