By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001.

Immunobiology: The Immune System in Health and Disease. 5th edition.
Show detailsMicroorganisms that cause pathology in humans and animals enter the body at different sites and produce disease by a variety of mechanisms. Many different infectious agents can cause pathology, and those that do are referred to as pathogenic microorganisms or pathogens. Invasions by microorganisms are initially countered, in all vertebrates, by innate defense mechanisms that preexist in all individuals and act within minutes of infection. Only when the innate host defenses are bypassed, evaded, or overwhelmed is an induced or adaptive immune response required. In the first part of this chapter we will describe briefly the infectious strategies of microorganisms before examining the innate host defenses that, in most cases, prevent infection from becoming established. Thus we will look at the defense functions of the epithelial surfaces of the body, the role of antimicrobial peptides and proteins, and the defense of body tissues by macrophages and neutrophils, which bind and ingest invading microorganisms in a process known as phagocytosis.
2-1. Infectious agents must overcome innate host defenses to establish a focus of infection
Our bodies are constantly exposed to microorganisms present in the environment, including infectious agents that have been shed from infected individuals. Contact with these microorganisms may occur through external or internal epithelial surfaces: the respiratory tract mucosa provides a route of entry for airborne microorganisms, the gastrointestinal mucosa for microorganisms in food and water; insect bites and wounds allow micro-organisms to penetrate the skin; and direct contact between individuals offers opportunities for infection of the skin and reproductive mucosa (Fig. 2.2).
Figure 2.2
Pathogens infect the body through a variety of routes.
In spite of this exposure, infectious disease is fortunately quite rare. The epithelial surfaces of the body serve as an effective barrier against most microorganisms, and are rapidly repaired if wounded. Furthermore, most of the microorganisms that do succeed in crossing the epithelial surfaces are efficiently removed by innate immune mechanisms that function in the underlying tissues. Thus in most cases these defenses, which we will examine in more detail in subsequent sections, prevent a site of infection from being established. It is difficult to know how many infections are repelled in this way, because there are no symptoms of disease. It is clear, however, that the microorganisms that a normal human being inhales or ingests, or that enter through minor wounds, are mostly held at bay or eliminated, since they seldom cause disease.
Infectious disease occurs when a microorganism succeeds in evading or overwhelming innate host defenses to establish a local site of infection and replication that allows its further transmission. In some cases, such as athlete’s foot, the initial infection remains local and does not cause significant pathology. In other cases the infectious agent causes significant pathology as it spreads through the lymphatics or the bloodstream, or as a result of secreting toxins.
Pathogen spread is often countered by an inflammatory response that recruits more effector molecules and cells of the innate immune system from local blood vessels (Fig. 2.3), while inducing clotting farther downstream so that pathogens cannot spread through the blood. The induced responses of innate immunity act over several days while an adaptive immune response gets underway in response to pathogen antigens delivered to local lymphoid tissue. Such a response can target specific features of the pathogen and will usually clear the infection and protect the host against reinfection with the same pathogen.
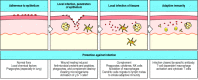
Figure 2.3
An infection and the response to it can be divided into a series of stages. These are illustrated here for an infectious microorganism entering through a wound in the skin. The infectious agent must first adhere to the epithelial cells and then cross (more...)
2-2. The epithelial surfaces of the body are the first defenses against infection
Our body surfaces are defended by epithelia, which provide a physical barrier between the internal milieu and the external world that contains pathogens. Epithelial cells are held together by tight junctions, which effectively form a seal against the external environment. Epithelia comprise the skin and the linings of the body’s tubular structures—the gastrointestinal, respiratory, and urinogenital tracts. Infections occur only when the pathogen can colonize or cross through these barriers, and since the dry, protective layers of the skin present a more formidable barrier, pathogen entry most often occurs through the internal epithelial surfaces. The importance of epithelia in protection against infection is obvious when the barrier is breached, as in wounds and burns, when infection is a major cause of mortality and morbidity. In the absence of wounding or disruption, pathogens normally cross epithelial barriers by binding to molecules on internal epithelial surfaces, or establish an infection by adhering to and colonizing these surfaces. This specific attachment allows the pathogen to infect the epithelial cell, or to damage it so that the epithelium can be crossed, or, in the case of colonizing pathogens, to avoid being dislodged by the flow of air or fluid across the epithelial surface. The internal epithelia are known as mucosal epithelia because they secrete a viscous fluid called mucus, which contains many glycoproteins called mucins. Microorganisms coated in mucus may be prevented from adhering to the epithelium, and in mucosal epithelia such as that of the respiratory tract, microorganisms can be expelled in the flow of mucus driven by the beating of epithelial cilia. The efficacy of mucus flow in clearing infection is illustrated by people with defective mucus secretion or inhibition of ciliary movement; they frequently develop lung infections caused by bacteria that colonize the epithelial surface. In the gut, peristalsis is an important mechanism for keeping both food and infectious agents moving through. Failure of peristalsis is typically accompanied by overgrowth of bacteria within the intestinal lumen.
Our surface epithelia are more than mere physical barriers to infection; they also produce chemical substances that are microbicidal or inhibit microbial growth (Fig. 2.4). For example, the antibacterial enzyme lysozyme is secreted in tears and saliva. The acid pH of the stomach and the digestive enzymes of the upper gastrointestinal tract create a substantial chemical barrier to infection. Further down the intestinal tract, antibacterial and antifungal peptides called cryptidins or α-defensins are made by Paneth cells, which are resident in the base of the crypts in the small intestine beneath the epithelial stem cells. Related antimicrobial peptides, the β-defensins, are made by other epithelia, primarily in the skin and respiratory tract. Such antimicrobial peptides play a role in the immune defense of many organisms, including insects. They are cationic peptides that are thought to kill bacteria by damaging the bacterial cell membrane. Another type of antimicrobial protein is secreted into the fluid that bathes the epithelial surfaces of the lung. This fluid contains two proteins—surfactant proteins A and D—that bind to and coat the surfaces of pathogens so that they are more easily phagocytosed by macrophages that have left the subepithelial tissues to enter the alveoli of the lung. Coating of a particle with proteins that facilitate its phagocytosis is known as opsonization and we will meet several examples of this defense strategy in this chapter.

Figure 2.4
Surface epithelia provide mechanical, chemical, and microbiological barriers to infection.
In addition to these defenses, most epithelial surfaces are associated with a normal flora of nonpathogenic bacteria that compete with pathogenic microorganisms for nutrients and for attachment sites on cells. The normal flora can also produce antimicrobial substances, such as the colicins (anti-bacterial proteins made by Escherichia coli) that prevent colonization by other bacteria. When the nonpathogenic bacteria are killed by antibiotic treatment, pathogenic microorganisms frequently replace them and cause disease.
2-3. After entering tissues, many pathogens are recognized, ingested, and killed by phagocytes
If a microorganism crosses an epithelial barrier and begins to replicate in the tissues of the host, it is, in most cases, immediately recognized by the mononuclear phagocytes, or macrophages, that reside in tissues. Macrophages mature continuously from circulating monocytes that leave the circulation to migrate into tissues throughout the body (see Fig. 1.3). They are found in especially large numbers in connective tissue, in association with the gastrointestinal tract, in the lung (where they are found in both the interstitium and the alveoli), along certain blood vessels in the liver (where they are known as Kupffer cells), and throughout the spleen, where they remove senescent blood cells. The second major family of phagocytes—the neutrophils, or polymorphonuclear neutrophilic leukocytes (PMNs or polys)—are short-lived cells that are abundant in the blood but are not present in normal, healthy tissues. Both these phagocytic cells have a key role in innate immunity because they can recognize, ingest, and destroy many pathogens without the aid of an adaptive immune response. Macrophages are the first to encounter pathogens in the tissues but they are soon re-inforced by the recruitment of large numbers of neutrophils to sites of infection.
Macrophages and neutrophils recognize pathogens by means of cell-surface receptors that can discriminate between the surface molecules displayed by pathogens and those of the host. These receptors, which we will examine in more detail later, include the macrophage mannose receptor, which is found on macrophages but not on monocytes or neutrophils, scavenger receptors, which bind many charged ligands, and CD14, a receptor for bacterial lipopolysaccharide (LPS) found predominantly on monocytes and macro-phages (Fig. 2.5). Pathogens can also interact with macrophages and neutrophils through receptors for complement borne on these cells. As we will see in the second part of the chapter, the complement system is activated rapidly in response to many types of infection, producing complement proteins that opsonize the surface of pathogens as they enter the tissues.

Figure 2.5
Phagocytes bear several different receptors that recognize microbial components and induce phagocytosis. The figure illustrates five such receptors on macrophages—CD14, CD11b/CD18 (CR3), the macrophage mannose receptor, the scavenger receptor, (more...)
Ligation of many of the cell-surface receptors that recognize pathogens leads to phagocytosis of the pathogen, followed by its death inside the phagocyte. Phagocytosis is an active process, in which the bound pathogen is first surrounded by the phagocyte membrane and then internalized in a membrane-bounded vesicle known as a phagosome, which becomes acidified. In addition to being phagocytic, macrophages and neutrophils have granules, called lysosomes, that contain enzymes, proteins, and peptides that can mediate an intracellular antimicrobial response. The phagosome fuses with one or more lysosomes to generate a phagolysosome in which the lysosomal contents are released to destroy the pathogen (see Fig. 2.5).
Upon phagocytosis, macrophages and neutrophils also produce a variety of other toxic products that help kill the engulfed microorganism (Fig. 2.6). The most important of these are hydrogen peroxide (H2O2), the superoxide anion (O2–), and nitric oxide (NO), which are directly toxic to bacteria. They are generated by lysosomal NADPH oxidases and other enzymes in a process known as the respiratory burst, as it is accompanied by a transient increase in oxygen consumption. Neutrophils are short-lived cells, dying soon after they have accomplished a round of phagocytosis. Dead and dying neutrophils are a major component of the pus that forms in some infections; bacteria that give rise to such infections are thus known as pyogenic bacteria. Macrophages, on the other hand, are long-lived and continue to generate new lysosomes. Patients with chronic granulomatous disease have a genetic deficiency of NADPH oxidase, which means that their phagocytes do not produce toxic oxygen derivatives and are less able to kill ingested microorganisms and clear the infection. People with this defect are unusually susceptible to bacterial and fungal infections, especially in infancy.

Figure 2.6
Bactericidal agents produced or released by phagocytes on the ingestion of microorganisms. Most of these agents are made by both macrophages and neutrophils. Some of them are toxic; others, such as lactoferrin, work by binding essential nutrients and (more...)
Macrophages can make this response immediately on encountering an infecting microorganism and this can be sufficient to prevent an infection from becoming established. The great cellular immunologist Elie Metchnikoff believed that the innate response of macrophages encompassed all host defense and, indeed, it is now clear that invertebrates, such as the sea star that he was studying, rely entirely on innate immunity for their defense against infection. Although this is not the case in humans and other vertebrates, the innate response of macrophages still provides an important front line of host defense that must be overcome if a microorganism is to establish an infection that can be passed on to a new host.
A key feature that distinguishes pathogenic from nonpathogenic micro-organisms is their ability to overcome innate immune defenses. Pathogens have developed a variety of strategies to avoid being immediately destroyed by macrophages. Many extracellular pathogenic bacteria coat themselves with a thick polysaccharide capsule that is not recognized by any phagocyte receptor. Other pathogens, for example mycobacteria, have evolved ways to grow inside macrophage phagosomes by inhibiting fusion with a lysosome. Without such devices, a microorganism must enter the body in sufficient numbers to simply overwhelm the immediate innate host defenses and establish a focus of infection.
The second important effect of the interaction between pathogens and tissue macrophages is activation of macrophages to release cytokines and other mediators that set up a state of inflammation in the tissue and bring neutrophils and plasma proteins to the site of infection. It is thought that the pathogen induces cytokine secretion by signals delivered through some of the receptors to which it binds, and we will see later how this occurs in response to LPS. Receptors that signal the presence of pathogens and induce cytokines also have another important role. This is to induce the expression of so-called co-stimulatory molecules on both macrophages and dendritic cells, another type of phagocytic cell present in tissues, thus enabling these cells to initiate an adaptive immune response (see Section 1-6).
The cytokines released by macrophages make an important contribution both to local inflammation and to other induced but nonadaptive responses that occur in the first few days of a new infection. We will be describing the role of individual cytokines in these induced responses in the last part of this chapter. However, since an inflammatory response is usually initiated within minutes of infection or wounding, we will outline here how it occurs and how it contributes to host defense.
2-4. Pathogen recognition and tissue damage initiate an inflammatory response
Inflammation plays three essential roles in combating infection. The first is to deliver additional effector molecules and cells to sites of infection to augment the killing of invading microorganisms by the front-line macrophages. The second is to provide a physical barrier preventing the spread of infection, and the third is to promote the repair of injured tissue, a nonimmunological role that we will not discuss further. Inflammation at the site of infection is initiated by the response of macrophages to pathogens.
Inflammatory responses are operationally characterized by pain, redness, heat, and swelling at the site of an infection, reflecting three types of change in the local blood vessels. The first is an increase in vascular diameter, leading to increased local blood flow—hence the heat and redness—and a reduction in the velocity of blood flow, especially along the surfaces of small blood vessels. The second change is that the endothelial cells lining the blood vessel are activated to express adhesion molecules that promote the binding of circulating leukocytes. The combination of slowed blood flow and induced adhesion molecules allows leukocytes to attach to the endothelium and migrate into the tissues, a process known as extravasation, which we will describe in detail later. All these changes are initiated by the cytokines produced by activated macrophages. Once inflammation has begun, the first cells attracted to the site of infection are generally neutrophils. They are followed by monocytes, which differentiate into more tissue macrophages. In the later stages of inflammation, other leukocytes such as eosinophils and lymphocytes also enter the infected site. The third major change in the local blood vessels is an increase in vascular permeability. Instead of being tightly joined together, the endothelial cells lining the blood vessel walls become separated, leading to exit of fluid and proteins from the blood and their local accumulation in the tissue. This accounts for the swelling, or edema, and pain—as well as the accumulation of plasma proteins that aid in host defense.
These changes are induced by a variety of inflammatory mediators released as a consequence of the recognition of pathogens. These include the lipid mediators of inflammation—prostaglandins, leukotrienes, and platelet-activating factor (PAF)—which are rapidly produced by macrophages through enzymatic pathways that degrade membrane phospholipids. Their actions are followed by those of the cytokines and chemokines (chemoattractant cytokines) that are synthesized and secreted by macrophages in response to pathogens. The cytokine tumor necrosis factor-α (TNF-α), for example, is a potent activator of endothelial cells.
As we will see in the next part of the chapter, another way in which pathogen recognition rapidly triggers an inflammatory response is through activation of the complement cascade. One of the cleavage products of the complement reaction is a peptide called C5a. C5a is a potent mediator of inflammation, with several different activities. In addition to increasing vascular permeability and inducing the expression of some adhesion molecules, it acts as a powerful chemoattractant for neutrophils and monocytes, and activates phagocytes and local mast cells, which are in turn stimulated to release granules containing the inflammatory molecule histamine and TNF-α.
If wounding has occurred, the injury to blood vessels immediately triggers two other protective enzyme cascades. The kinin system is an enzymatic cascade of plasma proteins that is triggered by tissue damage to produce several inflammatory mediators, including the vasoactive peptide bradykinin. This causes an increase in vascular permeability that promotes the influx of plasma proteins to the site of tissue injury. It also causes pain, which, although unpleasant to the victim, draws attention to the problem and leads to immobilization of the affected part of the body, which helps to limit the spread of any infectious agents. The coagulation system is another enzymatic cascade of plasma enzymes that is triggered following damage to blood vessels. This leads to the formation of a clot, which prevents any microorganisms from entering the bloodstream. Both these cascades have an important role in the inflammatory response to pathogens even if wounding or gross tissue injury has not occurred, as they are also triggered by endothelial cell activation. Thus, within minutes of the penetration of tissues by a pathogen, the inflammatory response causes an influx of proteins and cells that will control the infection. It also forms a physical barrier to limit the spread of infection and makes the host fully aware of what is going on.
Summary
The mammalian body is susceptible to infection by many pathogens, which must first make contact with the host and then establish a focus of infection in order to cause disease. These pathogens differ greatly in their lifestyles, the structures of their surfaces, and means of pathogenesis, which therefore requires an equally diverse set of defensive responses from the host immune system. The first phase of host defense consists of those mechanisms that are present and ready to resist an invader at any time. The epithelial surfaces of the body keep pathogens out, and protect against colonization and against viruses and bacteria that enter through specialized cell-surface interactions, by preventing pathogen adherence and by secreting antimicrobial enzymes and peptides. Bacteria, viruses, and parasites that overcome this barrier are faced immediately by tissue macrophages equipped with surface receptors that can bind and phagocytose many different types of pathogen. This, in turn, leads to an inflammatory response, which causes the accumulation of plasma proteins, including the complement components that provide circulating or humoral innate immunity, as will be described in the next part of the chapter, and phagocytic neutrophils at the site of infection. Innate immunity provides a front line of host defense through effector mechanisms that engage the pathogen directly, act immediately on contact with it, and are unaltered in their ability to resist a subsequent challenge with either the same or a different pathogen. These mechanisms often succeed in preventing an infection from becoming established. If not, they are reinforced through the recruitment and increased production of further effector molecules and cells in a series of induced responses that we will consider later in this chapter. These induced innate responses often fail to clear the infection. In that case, macrophages and other cells activated in the early innate response help to initiate the development of an adaptive immune response.
- Infectious agents must overcome innate host defenses to establish a focus of infection
- The epithelial surfaces of the body are the first defenses against infection
- After entering tissues, many pathogens are recognized, ingested, and killed by phagocytes
- Pathogen recognition and tissue damage initiate an inflammatory response
- Summary
- The front line of host defense - ImmunobiologyThe front line of host defense - Immunobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...