By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001.

Immunobiology: The Immune System in Health and Disease. 5th edition.
Show detailsHaving considered how an appropriate primary immune response is mounted to pathogens in both the peripheral lymphoid system and the mucosa-associated lymphoid tissues, we now turn to immunological memory, which is a feature of both compartments. Perhaps the most important consequence of an adaptive immune response is the establishment of a state of immunological memory. Immunological memory is the ability of the immune system to respond more rapidly and effectively to pathogens that have been encountered previously, and reflects the preexistence of a clonally expanded population of antigen-specific lymphocytes. Memory responses, which are called secondary, tertiary, and so on, depending on the number of exposures to antigen, also differ qualitatively from primary responses. This is particularly clear in the case of the antibody response, where the characteristics of antibodies produced in secondary and subsequent responses are distinct from those produced in the primary response to the same antigen. Memory T-cell responses have been harder to study, but can also be distinguished from the responses of naive or effector T cells. The principal focus of this section will be the altered character of memory responses, although we will also discuss emerging explanations of how immunological memory persists after exposure to antigen. A long-standing debate about whether specific memory is maintained by distinct populations of long-lived memory cells that can persist without residual antigen, or by lymphocytes that are under perpetual stimulation by residual antigen, appears to have been settled in favor of the former hypothesis.
10-21. Immunological memory is long-lived after infection or vaccination
Most children in the United States are now vaccinated against measles virus; before vaccination was widespread, most were naturally exposed to this virus and suffered from an acute, unpleasant, and potentially dangerous viral illness. Whether through vaccination or infection, children exposed to the virus acquire long-term protection from measles. The same is true of many other acute infectious diseases: this state of protection is a consequence of immunological memory.
The basis of immunological memory has been hard to explore experimentally. Although the phenomenon was first recorded by the ancient Greeks and has been exploited routinely in vaccination programs for over 200 years, it is just now becoming clear that memory reflects a persistent population of specialized memory cells that is independent of the continued persistence of the original antigen that induced them. This mechanism of maintaining memory is consistent with the finding that only individuals who were themselves previously exposed to a given infectious agent are immune, and that memory is not dependent on repeated exposure to infection as a result of contacts with other infected individuals. This was established by observations made on remote island populations, where a virus such as measles can cause an epidemic, infecting all people living on the island at that time, after which the virus disappears for many years. On reintroduction from outside the island, the virus does not affect the original population but causes disease in those people born since the first epidemic. This means that immunological memory need not be maintained by repeated exposure to infectious virus.
Instead, it is most likely that memory is sustained by long-lived antigen-specific lymphocytes that were induced by the original exposure and that persist until a second encounter with the pathogen. It was thought that retained antigen, bound in immune complexes on follicular dendritic cells, might be crucial in maintaining these cells, but recent experiments suggest otherwise. While most of the memory cells are in a resting state, careful studies have shown that a small percentage are dividing at any one time. What stimulates this infrequent cell division is unclear. However, cytokines such as those produced either constitutively or during the course of antigen-specific immune responses directed at noncross-reactive antigens could be responsible. One such cytokine, IL-15, has been implicated in maintaining CD8 memory T cells. Regardless of cell division, the number of memory cells for a given antigen is highly regulated, remaining practically constant during the memory phase.
Immunological memory can be measured experimentally in various ways. Adoptive transfer assays (seeAppendix I, Section A-42) of lymphocytes from animals immunized with simple, nonliving antigens have been favored for such studies, as the antigen cannot proliferate. When an animal is first immunized with a protein antigen, helper T-cell memory against that antigen appears abruptly and is at its maximal level after 5 days or so. Antigen-specific memory B cells appear some days later, because B-cell activation cannot begin until armed helper T cells are available, and B cells must then enter a phase of proliferation and selection in lymphoid tissue. By one month after immunization, memory B cells are present at their maximal levels. These levels are then maintained with little alteration for the lifetime of the animal. In these experiments, the existence of memory cells is measured purely in terms of the transfer of specific responsiveness from an immunized, or ‘primed,’ animal to an irradiated, immunoincompetent and nonimmunized recipient. In the following sections, we will look in more detail at the changes that occur in lymphocytes after antigen priming, and discuss the mechanisms that might account for these changes.
10-22. Both clonal expansion and clonal differentiation contribute to immunological memory in B cells
Immunological memory in B cells can be examined by isolating B cells from immunized mice and restimulating them with antigen in the presence of armed helper T cells specific for the same antigen. The response of these primed B cells can be compared with the primary B-cell response seen on isolating B cells from unimmunized mice and stimulating them with antigen in the same way (Fig. 10.24). By these means, it is possible to show that antigen-specific memory B cells differ both quantitatively and qualitatively from naive B cells. B cells that can respond to antigen increase in frequency after priming by about 10- to 100-fold (see Fig. 10.24) and produce antibody of higher average affinity than unprimed B lymphocytes; the affinity of that antibody continues to increase during the ongoing secondary and subsequent antibody responses (Fig. 10.25). The secondary antibody response is characterized in its first few days by the production of small amounts of IgM antibody and larger amounts of IgG antibody, with some IgA and IgE. These antibodies are produced by memory B cells that have already switched from IgM to these more mature isotypes and express IgG, IgA, or IgE on their surface, as well as a somewhat higher level of MHC class II molecules than is characteristic of naive B cells. Increased affinity for antigen and increased levels of MHC class II facilitate antigen uptake and presentation, and allow memory B cells to initiate their critical interactions with armed helper T cells at lower doses of antigen. Unlike memory T cells, which can traffic to tissues owing to changes in cell-surface molecules that affect migration and homing, it is thought that memory B cells continue to recirculate through the same secondary lymphoid compartments that contain naive B cells, principally the follicles of spleen, lymph node, and Peyer's patch. Some memory B cells can also be found in marginal zones, though it is not clear whether these represent a distinct subset of memory B cells.
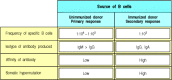
Figure 10.24
The generation of secondary antibody responses from memory B cells is distinct from the generation of the primary antibody response. These responses can be studied and compared by isolating B cells from immunized and unimmunized donor mice, and stimulating (more...)
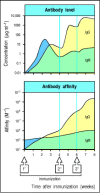
Figure 10.25
The affinity as well as the amount of antibody increases with repeated immunization. The upper panel shows the increase in the level of antibody with time after primary, followed by secondary and tertiary, immunization; the lower panel shows the increase (more...)
The distinction between primary and secondary antibody responses is most clearly seen in those cases where the primary response is dominated by antibodies that are closely related and show few if any somatic hypermutations. This occurs in inbred mouse strains in response to certain haptens that are recognized by a limited set of naive B cells. The antibodies produced are encoded by the same VH and VL genes in all animals of the strain, suggesting that these variable regions have been selected during evolution for recognition of determinants on pathogens that happen to cross-react with some haptens. As a result of the uniformity of these primary responses, changes in the antibody molecules produced in secondary responses to the same antigens are easy to observe. These differences include not only numerous somatic hypermutations in antibodies containing the dominant variable regions but also the addition of antibodies containing VH and VL gene segments not detected in the primary response. These are thought to derive from B cells that were activated at low frequency during the primary response, and thus not detected, but that differentiated into memory B cells.
10-23. Repeated immunizations lead to increasing affinity of antibody owing to somatic hypermutation and selection by antigen in germinal centers
Upon reexposure to the same antigen, a secondary immune response will ensue. In some ways, this resembles the primary immune response, with the initial proliferation of B cells and T cells at the interface between the T- and B-cell zones. The secondary response is characterized by early and vigorous generation of plasma cells, thus accounting for early profuse IgG production. Some B cells that have not yet undergone terminal differentiation can migrate into the follicle and become germinal center B cells. There, these B cells enter a second proliferative phase, during which the DNA encoding their immunoglobulin V domains again undergoes somatic hypermutation before the B cells differentiate into antibody-secreting plasma cells (see Section 9-7).
The antibodies produced by plasma cells in the primary and early secondary response have an important role in driving affinity maturation in the secondary response. In secondary and subsequent immune responses, any persisting antibodies produced by the B cells that differentiated in the primary response are immediately available to bind the newly introduced antigen. Some of these antibodies divert antigen to phagocytes for degradation and disposal (see Section 9-20). If there is sufficient preexisting antibody to clear or inactivate the pathogen, it is possible that no immune response will ensue. However, if there is a trace excess of antigen, B cells whose receptors bind the antigen with sufficient avidity to compete with the preexisting antibody will take up the uncomplexed antigen, process it into peptide fragments, and present these peptides, bound to MHC class II molecules, to armed helper T cells surrounding and infiltrating the germinal centers (see Section 9-8). Contact between the B cells presenting antigenic peptides and armed helper T cells specific for the same peptide leads to an exchange of activating signals and the rapid proliferation of both activated antigen-specific B cells and helper T cells. Thus, only the higher-affinity memory B cells are efficiently stimulated in the secondary immune response. In this way, the affinity of the antibody produced rises progressively, as only B cells with high-affinity antigen receptors can bind antigen efficiently and be driven to proliferate by antigen-specific helper T cells (Fig. 10.26).
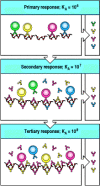
Figure 10.26
The mechanism of affinity maturation in an antibody response. At the beginning of a primary response, B cells with receptors of a wide variety of affinities (KA), most of which will bind antigen with low affinity, take up antigen, present it to helper (more...)
10-24. Memory T cells are increased in frequency and have distinct activation requirements and cell-surface proteins that distinguish them from armed effector T cells
Because the T-cell receptor does not undergo isotype switching or affinity maturation, memory T cells have been far more difficult to characterize than memory B cells. Furthermore, it has proved hard to distinguish between effector T cells and memory T cells on the basis of their phenotype. After immunization, the number of T cells reactive to a given antigen increases markedly as effector T cells are produced, and then falls back to persist at a level significantly (100- to 1000-fold) above the initial frequency for the rest of the animal's or person's life (Fig. 10.27). These cells carry cell-surface proteins more characteristic of armed effector cells than of naive T cells. However, they are long-lived cells with distinct properties in terms of surface molecule expression, response to stimuli, and expression of genes that control cell survival. Therefore, they should be specifically designated memory T cells. In the case of B cells, the distinction between effector and memory cells is more obvious and has been recognized for some time because effector B cells, as we saw in Chapter 9, are terminally differentiated plasma cells that have already been activated to secrete antibody until they die.
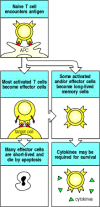
Figure 10.27
Encounter with antigen generates effector T cells and long-lived memory T cells. On priming with antigen, a naive T cell divides and differentiates. Most of the progeny are relatively short-lived effector cells. However, some become long-lived memory (more...)
A major problem in experiments aimed at establishing the existence of memory T cells is that most assays for T-cell effector function take several days, during which the putative memory T cells are reinduced to armed effector cell status. Thus, these assays do not distinguish preexisting effector cells from memory T cells. This problem does not apply to cytotoxic T cells, however, as cytotoxic effector T cells can program a target cell for lysis in 5 minutes. Memory CD8 T cells need to be reactivated to become cytotoxic, but they can do so without undergoing DNA synthesis, as shown by studies carried out in the presence of mitotic inhibitors. Recently, it has become possible to track particular clones of antigen-specific CD8 T cells by staining them with tetrameric MHC:peptide complexes (seeAppendix I, Section A-28). It has been found that the number of antigen-specific CD8 T cells increases dramatically during an infection, and then drops by up to 100-fold; nevertheless, this final level is distinctly higher than before priming. These cells continue to express some markers characteristic of activated cells, like CD44, but stop expressing other activation markers, like CD69. In addition, they express more Bcl-2, a protein that promotes cell survival and may be responsible for the long half-life of memory CD8 cells. These cells are more sensitive to restimulation by antigen than are naive cells, and more quickly and more vigorously produce cytokines such as IFN-γ in response to such stimulation.
The issue is more difficult to address for CD4 T-cell responses, and the identification of memory CD4 T cells rests largely, but not entirely, on the existence of a long-lived population of cells that have surface characteristics of activated armed effector T cells (Fig. 10.28) but that are distinct from them in that they require additional restimulation before acting on target cells. Changes in three cell-surface proteins—L-selectin, CD44, and CD45—are particularly significant after exposure to antigen. L-selectin is lost on most memory CD4 T cells, whereas CD44 levels are increased on all memory T cells; these changes contribute to directing the migration of memory T cells from the blood into the tissues rather than directly into lymphoid tissues. The isoform of CD45 changes because of alternative splicing of exons that encode the extracellular domain of CD45 (Fig. 10.29), leading to isoforms that associate with the T-cell receptor and facilitate antigen recognition. These changes are characteristic of cells that have been activated to become armed effector T cells (see Section 8-12), yet some of the cells on which these changes have occurred have many characteristics of resting CD4 T cells, suggesting that they represent memory CD4 T cells. Only after reexposure to antigen on an antigen-presenting cell do they achieve armed effector T-cell status, and acquire all the characteristics of armed TH2 or TH1 cells, secreting IL-4 and IL-5, or IFN-γ and TNF-β, respectively.

Figure 10.28
Many cell-surface molecules have altered expression on memory T cells. This is seen most clearly in the case of CD45, where there is a change in the isoforms expressed (see Fig. 10.29). Many of these changes are also seen on cells that have been activated (more...)
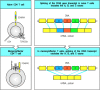
Figure 10.29
Memory CD4 T cells express altered CD45 isoforms that regulate the interaction of the T-cell receptor with its co-receptors. CD45 is a transmembrane tyrosine phosphatase with three variable exons (A, B, and C) that encode part of its external domain. (more...)
It thus seems reasonable to designate these cells as memory CD4 T cells, and to surmise that naive CD4 T cells can differentiate into armed effector T cells or into memory T cells that can later be activated to effector status. Recent experiments show that CD4 cells can differentiate into two types of memory cell, with distinct activation characteristics. One type are called effector memory cells because they can rapidly mature into effector CD4 T cells and secrete large amounts of IFN-γ, IL-4, and IL-5 early after restimulation. These cells lack the chemokine receptor CCR7 but express high levels of β-1 and β-2 integrins, as well as receptors for inflammatory chemokines. This profile suggests these effector memory cells are specialized for quickly entering inflamed tissues. The other type are called central memory cells. They express CCR7 and thus would be expected to recirculate more easily to T zones of secondary lymphoid tissues, as do naive T cells. These central memory cells are very sensitive to T-cell receptor cross-linking and quickly upregulate CD40L in response to it; however, they take longer to differentiate into effector cells and thus do not secrete as much cytokine as do effector memory cells early after restimulation. Interestingly, CD8 T cells can also be divided into analogous central and effector memory subsets.
As with memory CD8 T cells, the field will soon be revolutionized by direct staining of CD4 T cells with peptide:MHC class II oligomers (seeAppendix I, Section A-28). This technique allows one not only to identify antigen-specific CD4 T cells but also, using intracellular cytokine staining (seeAppendix I, Section A-27), to determine whether they are TH1 or TH2 cells. These improvements in the identification and phenotyping of CD4 T cells will rapidly increase our knowledge of these hitherto mysterious cells, and could contribute valuable information on naive, memory, and effector CD4 T cells.
10-25. In immune individuals, secondary and subsequent responses are mediated solely by memory lymphocytes and not by naive lymphocytes
In the normal course of an infection, a pathogen first proliferates to a level sufficient to elicit an adaptive immune response and then stimulates the production of antibodies and effector T cells that eliminate the pathogen from the body. Most of the armed effector T cells then die, and antibody levels gradually decline after the pathogen is eliminated, because the antigens that elicited the response are no longer present at the level needed to sustain it. We can think of this as feedback inhibition of the response. Memory T and B cells remain, however, and maintain a heightened ability to mount a response to a recurrence of infection with the same pathogen.
The antibody and memory T cells remaining in an immunized individual also prevent the activation of naive B and T cells by the same antigen. Such a response would be wasteful, given the presence of memory cells that can respond much more quickly. The suppression of naive lymphocyte activation can be shown by passively transferring antibody or memory T cells to naive recipients; when the recipient is then immunized, naive lymphocytes do not respond to the original antigen, but responses to other antigens are unaffected. This has been put to practical use to prevent the response of Rh- mothers to their Rh+ children; if anti-Rh antibody is given to the mother before she reacts to her child's red blood cells, her response will be inhibited. The mechanism of this suppression is likely to involve the antibody-mediated clearance and destruction of the child's red blood cells, thus preventing naive B cells and T cells from mounting an immune response. Memory B-cell responses are not inhibited by antibody against the antigen, so the Rh- mothers at risk must be identified and treated before a response has occurred. This is because memory B cells are much more sensitive, because of their high affinity and alterations in their B-cell receptor signaling requirements, to smaller amounts of antigen that cannot be efficiently cleared by the passive anti-Rh antibody. The ability of memory B cells to be activated to produce antibody even when exposed to preexisting antibody also allows secondary antibody responses to occur in individuals who are already immune.
Adoptive transfer of immune T cells (seeAppendix I, Section A-42) to naive syngeneic mice also prevents the activation of naive T cells by antigen. This has been shown most clearly for cytotoxic T cells. It is possible that, once reactivated, the memory CD8 T cells regain cytotoxic activity sufficiently rapidly to kill the antigen-presenting cells that are required to activate naive CD8 T cells, thereby inhibiting the latter's activation.
These mechanisms might also explain the phenomenon known as original antigenic sin. This term was coined to describe the tendency of people to make antibodies only to epitopes expressed on the first influenza virus variant to which they are exposed, even in subsequent infections with variants that bear additional, highly immunogenic, epitopes (Fig. 10.30). Antibodies against the original virus will tend to suppress responses of naive B cells specific for the new epitopes. This might benefit the host by using only those B cells that can respond most rapidly and effectively to the virus. This pattern is broken only if the person is exposed to an influenza virus that lacks all epitopes seen in the original infection, as now no preexisting antibodies bind the virus and naive B cells are able to respond.

Figure 10.30
When individuals who have already been infected with one variant of influenza virus are infected with a second variant they make antibodies only to epitopes that were present on the initial virus. A child infected for the first time with an influenza (more...)
Summary
Protective immunity against reinfection is one of the most important consequences of adaptive immunity operating through the clonal selection of lymphocytes. Protective immunity depends not only on preformed antibody and armed effector T cells, but most importantly on the establishment of a population of lymphocytes that mediate long-lived immunological memory. The capacity of these cells to respond rapidly to restimulation with the same antigen can be transferred to naive recipients by primed B and T cells. The precise changes that distinguish naive, effector, and memory lymphocytes are now being characterized and, with the advent of receptor-specific reagents, the relative contributions of clonal expansion and differentiation to the memory phenotype are rapidly being clarified. Memory B cells can also be distinguished by changes in their immunoglobulin genes because of isotype switching and somatic hypermutation, and secondary and subsequent immune responses are characterized by antibodies with increasing affinity for the antigen.
- Immunological memory is long-lived after infection or vaccination
- Both clonal expansion and clonal differentiation contribute to immunological memory in B cells
- Repeated immunizations lead to increasing affinity of antibody owing to somatic hypermutation and selection by antigen in germinal centers
- Memory T cells are increased in frequency and have distinct activation requirements and cell-surface proteins that distinguish them from armed effector T cells
- In immune individuals, secondary and subsequent responses are mediated solely by memory lymphocytes and not by naive lymphocytes
- Summary
- Immunological memory - ImmunobiologyImmunological memory - Immunobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...