Selection of Primary Studies
The initial literature search returned 756 records (Appendix 2). An additional 61 citations were identified through grey literature searching. Of these, 119 records were considered potentially relevant. The full text of two records could not be located,11,12 and 117 records were examined in full-text format. After full-text review, 98 records were excluded. In total, 21 records were included (representing 19 unique systematic reviews).13-33 The full lists of included and excluded records are available in Appendix 3 and Appendix 4.
Of the 19 included reviews, 11 provided usable data to address research questions 1 to 4. No usable data were reported from eight reviews;15,20,21,25-28,31 these reviews remained included, but no data were extracted.
Five systematic reviews20,25-27,31 had a search date before publication of the DAPT trial.34 Because this was the largest RCT to investigate the optimal duration of DAPT, we extracted data only from systematic reviews that (a) performed their literature search after publication of the DAPT trial and (b) included the DAPT trial. One additional review was performed after publication of the DAPT trial, but the DAPT trial was not included;28 no data were included from this review.
Two additional systematic reviews15,21 met the inclusion criteria but did not provide data for a time comparison of interest.
Thus, our evidence synthesis was based on data reported in 11 unique systematic reviews ().13,14,16-19,22,23,29,30,33
Summary of Systematic Reviews Included in the Evidence Synthesis.
Study and Patient Characteristics
Most of the included systematic reviews aimed to look at the optimal duration of DAPT, thus including RCTs that compared any duration of DAPT. As such, most systematic reviews included RCTs that were beyond the scope of this review (e.g., three months versus 12 months DAPT). Data to address our research questions were taken from subgroup analyses of the systematic reviews or from RCTs that compared six to 12 months of DAPT with DAPT for more than 12 months ().
Randomized Controlled Trials Included in the Systematic Reviews.
Of the RCTs included in the relevant systematic reviews, seven RCTs involved a time comparison of interest (6 to 12 months versus > 12 months).34-40 The remaining RCTs involved comparison of short-term DAPT durations (e.g., three months or six months versus 12 months).41-45 These comparisons were outside the scope of our review, and no data were extracted from these RCTs. Of note, this approach was motivated by the policy context for this review. Because some jurisdictional drug plans currently limit coverage of P2Y12 inhibitors to 12 months, only assessing the impact of treating for a longer period is relevant to inform policy work.
Two of the RCTs included in most of the systematic reviews included participants with an implanted BMS (PRODIGY,38 DAPT trial34). Each of the included systematic reviews stated that they aimed to investigate the optimal duration of DAPT in patients with an implanted DES; however, most did not exclude the BMS patients from the PRODIGY trial in their analysis.13,17-19,23,30 (About 25% of patients in the PRODIGY trial received a BMS.38) Of the included reviews, three22,29,33 excluded PRODIGY38 trial patients with a BMS in their analyses.
Although the DAPT trial34 also randomized patients who had received a BMS, the primary publication of the trial data excluded the BMS patients. Therefore, only DAPT trial patients with a DES were included in most of the included systematic reviews.
Most of the included systematic reviews did not specify the generation of DES of interest, although one review included only RCTs that assessed duration of DAPT among patients who had received a second-generation stent,16 and one stated that it focused on “newer generation stents” without defining “newer generation.”33
Of the included reviews, four were based in China, with three from author groups based in the US (). The funding sources of the reviews were not well reported, with 45% (5/11) not reporting the source of funding. Of those that did report the funding source, three received no funding, two were funded by non-pharmaceutical sources, and one reported receiving “financial support for the research, authorship, and/or publication of the article” from multiple pharmaceutical companies ().
The included systematic reviews included a common set of five RCTs that met our inclusion criteria.34-38 Two recent systematic reviews13,33 also included an RCT published in 2016 (OPTIDUAL39), and one systematic review18 included data from an RCT (Hu 201240) published only in abstract form. The most recent search date was January 28, 2016.
The main inclusion and exclusion criteria for the RCTs that met our inclusion criteria are presented in .
Major Inclusion and Exclusion Criteria of Included Randomized Controlled Trials.
The characteristics of the patients in each RCT, including the types of stents implanted, are reported in .
Characteristics of Patients Included in the Randomized Controlled Trials.
The mean age of the included participants was more than 60 years in each RCT (). Most of the participants were male (69% to 81%), and about one-third of participants in each RCT had diabetes (24% to 38%). There was wide variation in the percentage of participants with ACS within the RCTs: Between 0.1% and 33.3% of participants had STEMI, between 7.1% and 22.9% of participants had non-STEMI, and between 9.1% and 38.6% of participants had unstable angina (). Two RCTs did not report the number of patients with ACS.35,40 Three trials reported the percentage of participants with a complex lesion (class B2 or C; 43% to 79%).34,36,38
Acute Coronary Syndrome Among Patients in the Randomized Controlled Trials.
Critical Appraisal of Included Studies
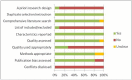
The AMSTAR checklist9 was applied to the 11 included systematic reviews with usable data (Appendix 5).
Most of the included systematic reviews scored 6 or 7 points on the AMSTAR scale (out of 11 points), while one review scored 9 points,16 and two scored 5 points.18,33 Only one review13 reported having an a priori research design, registered with the PROSPERO database. None of the included systematic reviews reported the sources of support for both the review and the included RCTs. All reviews reported using duplicate reviewers for selection or data extraction, and most performed a comprehensive literature search (). We were unable to judge the quality of the literature search performed by one review33 because insufficient details were reported; however, this review located the same RCTs as the reviews that used a comprehensive search strategy. Although all reviews addressed the quality of the included RCTs, few reviews commented on the quality of the RCTs in their publication or incorporated quality into their meta-analysis. However, the included RCTs were judged by most of the systematic reviews to be at low risk of bias, except for the category “blinding of participants and personnel,” because most of the included RCTs were open label. No data were excluded from our analysis on the basis of the AMSTAR assessment.
Proportion of Reviews That Fulfilled Each AMSTAR Criterion.
We were unable to judge whether two reviews22,30 used appropriate methods to pool data. Both reported that they pooled data using fixed-effects and random-effects models; however, neither study reported which model was used to create their published data. The remaining reviews reported the results of fixed-effects or random-effects meta-analysis. Heterogeneity was minor (0% to 40%) for most outcomes, and there was little difference between fixed-effects and random-effects estimates in the studies that reported both.33
Data Summary: Research Question 1
What are the benefits and harms of extended DAPT (> 12 months) with ASA plus a P2Y12 inhibitor (clopidogrel, prasugrel, ticagrelor) compared with DAPT of a shorter duration (6 to 12 months) following PCI with stenting?
In total, 11 systematic reviews addressed research question 1.13,14,16-19,22,23,29,30,33
Although each of the included systematic reviews reported that they aimed to evaluate allcause death among patients with an implanted DES, two of the included RCTs also involved patients with BMS (DAPT,34 PRODIGY38). Few systematic reviews excluded patients with BMS from their analysis. Therefore, the data used to address research question 1 are from a population of DES and BMS patients, unless otherwise noted.
All-Cause Death
In total, 11 systematic reviews assessed all-cause death with different durations of DAPT.13,14,16-19,22,23,29,30,33 Comparisons included six months versus more than 12 months, six to 12 months versus more than 12 months, and 12 months versus more than 12 months. One publication included network meta-analyses that assessed all-cause death associated with different durations of extended DAPT (24 months, 30 months, and 36 months).29
Six Months Versus More Than 12 Months
Five systematic reviews assessed all-cause death reported in RCTs that compared six months of DAPT with extended DAPT (> 12 months).13,16,17,22,29 Of these, four performed a meta-analysis, directly comparing six months with more than 12 months.13,16,17,22 Each review included only one RCT for this time comparison (PRODIGY38), with a duration of six months compared with 24 months.
Each of reviews that compared six months with more than 12 months of DAPT reported no statistically significant difference in the odds or risk of all-cause death between durations (). One review16 reported no significant difference in the odds of all-cause death among patients with a second-generation DES (OR 1.11; 95% confidence interval [CI], 0.35 to 3.57) (), with high heterogeneity (I2 = 75%) between everolimus-eluting and zotarolimus-eluting stents.
Summary of Evidence Available for All-Cause Death: 6 Months Versus >12 Months.
One systematic review29 compared all durations of DAPT via network meta-analysis involving patients who had undergone PCI with a DES. This analysis involved direct and indirect evidence from four RCTs having a total of 17,743 patients.34-36,38 In their analysis, Xie et al.29 found no significant difference in the odds of all-cause death between six months and 24, 30, or 36 months of DAPT ().
Summary of Evidence Available for All-Cause Death: Network Meta-Analysis, 6 Months Versus >12 Months.
Six to 12 Months Versus More Than 12 Months
Three systematic reviews18,19,33 assessed all-cause death associated with six to 12 months of DAPT compared with extended DAPT (> 12 months). All three reviews included ARCTIC-Interruption, DAPT, DES-LATE, ITALIC, and PRODIGY. One review18 also included an RCT reported only in abstract format,40 and one review33 included a recently published RCT (OPTIDUAL39).
All three systematic reviews reported no statistically significant difference in the odds of all-cause death between six to 12 months of DAPT and more than 12 months of DAPT ().18,19,33
Summary of Evidence Available for All-Cause Death: 6 to 12 Months Versus > 12 Months.
Twelve Months Versus More Than 12 Months
Seven systematic reviews compared all-cause death among patients receiving DAPT for 12 months with those receiving DAPT for more than 12 months.13,14,16,17,22,23,30 Most of the reviews included three RCTS (DES-LATE,36 ARCTIC-Interruption,35 DAPT34). One review13 also included a recently published RCT (OPTIDUAL39) that compared 12 months and 30 months of DAPT. One review16 included only second-generation DESs from one RCT (DES-LATE36).
In total, six systematic reviews reported increased odds or risk of all-cause death among patients receiving DAPT for more than 12 months compared with those who received DAPT for 12 months (). Among patients with a second-generation DES, one review16 reported no statistically significant difference in all-cause death between 12 months and more than 12 months of DAPT ().
Summary of Evidence Available for All-Cause Death: 12 Months Versus > 12 Months.
One systematic review29 compared all durations of DAPT via network meta-analysis. Xie et al.29 reported no statistically significant difference in the odds of all-cause death between 12 months of DAPT and 24, 30, or 36 months of DAPT among patients with a DES ().
Summary of Evidence Available for All-Cause Death: Network Meta-Analysis, 12 Months Versus > 12 Months.
Longer Than 12 Months
One systematic review29 compared all durations of DAPT via network meta-analysis. Xie et al.29 reported no statistically significant difference in the odds of all-cause death between 24 months of DAPT and 30 or 36 months of DAPT or between 30 months of DAPT and 36 months ().
Summary of Evidence Available for All-Cause Death: Network Meta-Analysis, > 12 Months Versus > 12 Months.
Cardiovascular Death
In total, nine systematic reviews assessed cardiovascular death with different durations of DAPT.13,14,16-19,22,23,30 Comparisons included six months versus more than 12 months, six to 12 months versus more than 12 months, and 12 months versus more than 12 months.
The reviews did not individually define “cardiovascular death.” Some review authors stated that they included cardiovascular death as defined in the included RCTs (Appendix 6).
Six Months Versus More Than 12 Months
Four systematic reviews compared cardiovascular death among patients who received six months of DAPT with those who received more than 12 months of DAPT.13,16,17,22 Each review included only one RCT for this time comparison (PRODIGY38), with a duration of six months compared with 24 months.
Each review found no statistically significant difference in the odds or risk of cardiovascular death between six months and more than 12 months of DAPT ().
Summary of Evidence Available for Cardiovascular Death: 6 Months Versus > 12 Months.
Six to 12 Months Versus More Than 12 Months
Two systematic reviews assessed cardiovascular death among patients who received six to 12 months of DAPT compared with those who received more than 12 months of DAPT.18,19 Four RCTs (DAPT,34 DES-LATE,36 ITALIC,37 PRODIGY38) were included in both reviews. Verdoia et al. also included data from the RCT by Hu et al.,40 which was reported in abstract form only.
Both reviews reported no statistically significant difference in the odds of cardiovascular death between six to 12 months of DAPT and more than 12 months of DAPT ().
Summary of Evidence Available for Cardiovascular Death: 6 to 12 Months Versus > 12 Months.
Twelve Months Versus More Than 12 Months
Seven systematic reviews assessed cardiovascular death among patients who received 12 months of DAPT compared with those who received more than 12 months of DAPT.13,14,16,17,22,23,30
Five reviews included both the DAPT34 and DES-LATE36 trials, while one review also included OPTIDUAL.39 One review16 that aimed to assess the optimal DAPT duration among patients who received a second-generation DES included only data from the DES-LATE trial.36
Among patients with an implanted DES, each of the reviews reported that there was no statistically significant difference in cardiovascular death between 12 months and more than 12 months of DAPT (via OR, relative risk, or hazard ratio [HR]; ). Among patients who received a second-generation DES, there was no significant difference in the odds of cardiovascular death between 12 months of DAPT and more than 12 months of DAPT ().
Summary of Evidence Available for Cardiovascular Death: 12 Months Versus > 12 Months.
Noncardiovascular Death
One systematic review22 reported the risk of noncardiovascular death among patients randomized to short or extended DAPT ().
Summary of Evidence Available for Noncardiovascular Death.
Among patients with an implanted DES, there was no significant difference in the risk of noncardiovascular death between those who received DAPT for six months and those who received DAPT for more than 12 months (). In contrast, there was a significant increase in the risk of noncardiovascular death among those who received DAPT for more than 12 months compared with those who received DAPT for 12 months (HR 1.89; 95% CI, 1.27 to 2.78) ().
Myocardial Infarction
In total, 11 systematic reviews assessed the impact of DAPT duration on myocardial infarction.13,14,16-19,22,23,29,30,33 Each of the reviews either did not report the definition of myocardial infarction or reported that they used the definitions of myocardial infarction used in the primary RCTs (Appendix 6).
Six Months Versus More Than 12 Months
Three systematic reviews13,16,17 reported the odds of myocardial infarction for six months of DAPT compared with extended DAPT. Each review included data from one trial for this comparison (PRODIGY38), including patients with a DES or BMS.
Among patients with a DES or BMS, two reviews reported no statistically significant difference in the odds of myocardial infarction between participants randomized to six months or more than 24 months ().13,17 Among patients with a second-generation DES, one review16 reported no significant difference in the odds of myocardial infarction between six months of DAPT and more than 12 months of DAPT ().
Summary of Evidence Available for Myocardial Infarction: 6 Months Versus > 12 Months.
One systematic review29 compared all durations of DAPT via network meta-analysis among patients with a DES. In their analysis, Xie et al.29 found no significant difference in the odds of myocardial infarction between six months of DAPT and 24, 30, or 36 months ().
Summary of Evidence Available for Myocardial Infarction: Network Meta-Analysis, 6 Months Versus > 12 Months.
Six to 12 Months Versus More Than 12 Months
Three systematic reviews assessed myocardial infarction among patients who received DAPT for six to 12 months compared with more than 12 months.18,19,33 Each review included the same five RCTs (DAPT, DES-LATE, ITALIC, PRODIGY, ARCTIC-Interruption), while one review18 also included an RCT reported only in abstract form (Hu et al.40) (). Each of the included reviews reported that the odds of myocardial infarction were significantly lower among patients who received DAPT for at least 12 months compared with patients who received DAPT for six to 12 months (). Of note, two of these reviews included a mixed population of patients with a BMS or DES,18,19 while one included only patients with a DES.33
Summary of Evidence Available for Myocardial Infarction: 6 to 12 Months Versus > 12 Months.
Twelve Months Versus More Than 12 Months
Eight systematic reviews13,14,16,17,22,23,29,30 assessed myocardial infarction among patients who received DAPT for 12 months compared with more than 12 months. Of these, two compared different durations of DAPT via meta-analysis.22,29 while the others compared 12 months with more than 12 months via meta-analysis.
Among patients with a DES, five reviews reported that the odds of myocardial infarction were significantly lower among patients who received DAPT for more than 12 months compared with 12 months (). In one review, there was no difference in the odds of myocardial infarction among patients with a second-generation DES ().
Summary of evidence available for myocardial infarction: 12 v. > 12 months.
Two of the included reviews22,29 used network meta-analyses to assess the optimal duration of DAPT among patients with a DES.
Compared with DAPT for 12 months, Palmerini et al.22 reported that the odds of a myocardial infarction were significantly lower among patients who received DAPT for more than 12 months (HR 0.59; 95% CI, 0.48 to 0.71) (). Among patients who received DAPT for more than 12 months, Xie et al.29 found no significant differences in the odds of myocardial infarction between 24 months and 30 or 36 months or between 30 months and 36 months ().
Summary of Evidence Available for Myocardial Infarction: Network Meta-Analysis.
Stroke
Four systematic reviews assessed the impact of DAPT duration on stroke.13,14,17,30 Data were available comparing either six months or 12 months of DAPT with DAPT extended for more than 12 months.
None of the included reviews reported the definition of stroke used. The definitions of stroke used in the primary RCTs are reported in Appendix 6.
Six Months Versus More Than 12 Months
Two systematic reviews13,17 assessed stroke among patients who received DAPT for six months compared with more than 12 months. Both reviews involved only data from the PRODIGY38 trial. Among patients with a DES or BMS, both reviews reported no significant difference in the odds of stroke between six months and more than 12 months of DAPT ().
Summary of Evidence Available for Stroke: 6 Months Versus > 12 Months.
Six to 12 Months Versus More Than 12 Months
None of the included systematic reviews assessed stroke among patients with a DES or BMS who received DAPT for six to 12 months compared with more than 12 months following stenting.
Twelve Months Versus More Than 12 Months
Four systematic reviews13,14,17,30 assessed stroke among patients with a DES who received DAPT for 12 months compared with more than 12 months. Each of the reviews included the same three RCTs (DAPT,34 DES-LATE,36 ARCTIC-Interruption35), with the most recently published review including one additional RCT (OPTIDUAL39). Among patients with a DES, each review reported no statistically significant difference in the odds or risk of stroke between patients who received DAPT for more than 12 months compared with those who received DAPT for 12 months ().
Summary of Evidence Available for Stroke: 12 Months Versus > 12 Months.
Stent Thrombosis
In total, 11 systematic reviews assessed stent thrombosis with different durations of DAPT.13,14,16-19,22,23,29,30,33 Comparisons included six months versus more than 12 months, six to 12 months versus more than 12 months, and 12 months versus more than 12 months. All of the included trials used the Academic Research Consortium criteria for defining stent thrombosis.46
Six Months Versus More Than 12 Months
Three systematic reviews assessed stent thrombosis among patients who received DAPT for six months compared with more than 12 months. Each review combined definite and probable stent thrombosis for this outcome, and each included data from the PRODIGY trial.38
Among patients with a DES or BMS, two reviews13,17 found no significant difference in the odds of probable or definite stent thrombosis between patients who received six months of DAPT or more than 12 months of DAPT (). Among patients who received a secondgeneration DES, one review16 reported no statistically significant difference between groups; however, the estimate was associated with a wide confidence interval (OR 2.50; 95% CI, 0.33 to 20.00) ().
Summary of Evidence Available for Stent Thrombosis: 6 Months Versus > 12 Months.
One review29 compared six months of DAPT with 24, 30, or 36 months of DAPT via network meta-analysis. Where six months of DAPT was compared with 24 or 36 months, Xie et al.29 reported no statistically significant difference in the odds of stent thrombosis (). However, the authors reported that 30 months of DAPT was associated with statistically lower odds of stent thrombosis compared with six months of DAPT ().
Summary of Evidence Available for Stent Thrombosis: Network Meta-Analysis, 6 Months Versus > 12 Months.
Six to 12 Months Versus More Than 12 Months
Three systematic reviews assessed stent thrombosis among patients who received DAPT for six to 12 months compared with more than 12 months.18,19,33 It was not well defined within each review whether the outcome was definite, probable, or definite/probable stent thrombosis.
Each review included the same five RCTs (DAPT,34 DES-LATE,36 ITALIC,37 PRODIGY,38 ARCTIC-Interruption35), while one review18 also included an RCT reported only in abstract form (Hu et al.40) (). Each reported that the odds of stent thrombosis were significantly lower among patients with a DES who received DAPT for at least 12 months compared with patients who received DAPT for six to 12 months ().
Summary of Evidence Available for Stent Thrombosis: 6 to 12 Months Versus > 12 Months.
Twelve Months Versus More Than 12 Months
Six systematic reviews assessed stent thrombosis among patients who received DAPT for 12 months compared with more than 12 months.13,14,16,17,23,30 Each review assessed definite/probable stent thrombosis; as well, Navarese et al.23 assessed definite stent thrombosis and very late stent thrombosis (stent thrombosis occurring more than one year after PCI) ().
Summary of Evidence Available for Stent Thrombosis: 12 Months Versus > 12 Months.
Among patients with a DES, five reviews13,14,17,23,30 reported that the odds of probable or definite stent thrombosis were significantly lower among patients who received DAPT for at least 12 months compared with patients who received DAPT for 12 months ().
Similarly, one review23 reported that the odds of definite stent thrombosis was significantly lower among patients who received more than 12 months of DAPT compared with those who received 12 months of DAPT (OR 0.30; 95% CI, 0.19 to 0.49) (). Navarese et al.23 also reported significantly lower odds of very late stent thrombosis among patients who received more than 12 months of DAPT (OR 0.33; 95% CI, 0.21 to 0.51) ().
Among patients with a second-generation DES, one review16 reported no statistically significant difference in probable or definite stent thrombosis between 12 months and more than 12 months of DAPT (OR 0.54; 95% CI, 0.87 to 1.02) ().
Via network meta-analysis, Palmerini et al.22 found a significantly lower risk of probable or definite stent thrombosis among patients who received more than 12 months of DAPT compared with 12 months of DAPT (). The numbers of trials and participants that informed this comparison were not reported. One review29 compared 12 months of DAPT with 24, 30, or 36 months of DAPT via network meta-analysis. Where 12 months of DAPT was compared with 24 or 36 months, Xie et al.29 reported no significant difference in the odds of stent thrombosis. However, the authors reported that 30 months of DAPT was associated with a statistically lower odds of stent thrombosis compared with 12 months of DAPT (OR 0.25; 95% CI, 0.09 to 0.99) ().
Summary of Evidence Available for Stent Thrombosis: Network Meta-Analysis, 12 Months Versus > 12 Months.
Longer Than 12 Months
Xie et al.29 compared the odds of stent thrombosis among treatment durations longer than 12 months via network meta-analysis. Among patients with a DES, the authors found no statistically significant difference in the odds of stent thrombosis between 24 months and 30 months, between 24 months and 36 months, or between 30 months and 36 months ().
Summary of Evidence Available for Stent Thrombosis: Network Meta-Analysis, > 12 Months.
Urgent Target Revascularization
None of the included systematic reviews assessed urgent revascularization. This outcome was reported in two of the included RCTs (ITALIC37, ARCTIC-Interruption35); findings are summarized in . There were no significant differences in risk between short and extended DAPT in either trial.
Summary of Evidence Available for Urgent Target Revascularization.
Major Adverse Cardiovascular Events
Two systematic reviews assessed major adverse cardiovascular events associated with different durations of DAPT.23,33 One review compared six to 12 months of DAPT with more than 12 months33 and one compared 12 months of DAPT with more than 12 months of DAPT.23
The components of the MACCE (major adverse cardiac and cerebrovascular event) outcome differed in each included RCT (). As such, it is difficult to draw conclusions from meta-analyses of these data.
Definitions of MACCE Used in Included Randomized Controlled Trials.
Despite differences in definitions, both Bittl et al.33 and Navarese et al.23 reported significantly lower odds of an event among patients with a DES who received more than 12 months of DAPT compared with a shorter duration ().
Summary of Evidence Available for MACCE.
Bleeding
Major Bleeding
In total, 11 systematic reviews assessed major bleeding associated with different durations of DAPT.13,14,16-19,22,23,29,30,33
Each of the underlying RCTs used a different classification system for bleeding (), and it is not appropriate to combine all bleeding data via meta-analysis.10 As such, we report the data separately for BARC, TIMI, and GUSTO classification systems. None of the eligible RCTs assessed bleeding by use of the REPLACE system.
Bleeding Criteria Used in Included Randomized Controlled Trials.
Three systematic reviews assessed bleeding by use of TIMI criteria,13,17,23 and one used BARC criteria (type 3 and 5).16 One additional review30 stated that they assessed TIMI major bleeding; however, the bleeding event counts included from the DAPT trial were assessed by use of GUSTO criteria; these data are not included in our analysis.
Compared with six months of DAPT, two reviews reported increased odds of TIMI major bleeding with more than 12 months of DAPT (). Both reviews included only one trial (PRODIGY38), in which about 25% of patients had a BMS.
Summary of Evidence Available for Major Bleeding.
Among participants with a second-generation DES, D’Ascenzo et al.16 reported that more than 12 months of DAPT was associated with increased odds of BARC type 3 or type 5 bleeding ().
One review reported that there was no statistically significant increase in the odds of TIMI major bleeding (OR 1.60; 95% CI, 0.97 to 2.64) among patients who received more than 12 months of DAPT compared with 12 months.
None of the included reviews assessed major bleeding by use of the GUSTO classification system.
Minor Bleeding
None of the included systematic reviews assessed minor bleeding.
Gastrointestinal Bleeding
None of the included systematic reviews or RCTs assessed gastrointestinal bleeding.
Data Summary: Research Question 2
Are there subgroups of patients for whom the optimal duration of DAPT with ASA plus a P2Y12 inhibitor (clopidogrel, prasugrel, ticagrelor) is different?
Limited data were available from the included systematic reviews to address this question: One review23 assessed outcomes among patients with and without ACS and for patients in different age groups (< 62.5 years versus > 62.5 years). The outcomes assessed in this review were cardiovascular death, myocardial infarction, and stent thrombosis.
Among patients with ACS, Navarese et al.23 reported no significant differences in the odds of cardiovascular death, myocardial infarction, or stent thrombosis between DAPT for 12 months and DAPT for longer than 12 months (). It was unclear which RCTs contributed data to the analysis and which stent types were included.
Summary of Evidence Available: Acute Coronary Syndrome.
Among patients without ACS, the authors found no significant differences in cardiovascular death or myocardial infarction between DAPT for 12 months and DAPT for longer than 12 months. DAPT for longer than 12 months was associated with significantly lower odds of stent thrombosis compared with DAPT for 12 months among patients without ACS ().
Among patients aged less than 62.5 years, Navarese et al.23 reported no significant differences in the odds of cardiovascular death, but lower odds of myocardial infarction and stent thrombosis between DAPT for 12 months and DAPT for longer than 12 months ().
Summary of Evidence Available: Age.
Among patients aged more than 62.5 years, there were no significant differences in cardiovascular death, myocardial infarction, or stent thrombosis between DAPT for 12 months and DAPT for longer than 12 months ().
Because limited data were available from the included systematic reviews and the data available were not well reported (e.g., it was unclear which RCTs contributed data to the analysis and which stent types were included), we sought data from eligible RCTs to address this research question.
Subgroup data were available from four RCTs (DAPT,34 ARCTIC-Interruption,35 ITALIC,37 PRODIGY38) to address the optimal duration of DAPT among one of the a priori—defined subgroups of interest. These data are summarized below.
Acute Coronary Syndrome
Subgroup data for ACS were available from the included RCTs for three outcomes: stent thrombosis, MACCE, and bleeding.
Stent Thrombosis
One trial (PRODIGY38) reported stent thrombosis among patients with ACS (). Valgimigli et al.38 reported no significant difference in the risk of definite stent thrombosis or definite/probable stent thrombosis between patients with ACS randomized to six months or 24 months of DAPT. No data were reported for patients without ACS.
Summary of Randomized Controlled Trials That Reported Stent Thrombosis in Patients With Acute Coronary Syndrome.
Major Adverse Cardiac and Cerebrovascular Events
Three RCTs reported MACCE among patients with and without ACS (PRODIGY, ARCTIC-Interruption, DES-LATE).35,36,38 Each used a different definition of MACCE ().
Despite differences in the outcome definition, each RCT found no significant difference in the risk of MACCE between six to 12 months of DAPT and more than 12 months of DAPT among patients either with or without ACS ().
Summary of Evidence Available for MACCE Among Patients With or Without Acute Coronary Syndrome.
Bleeding
One trial (PRODIGY38) reported bleeding among patients with ACS or stable coronary artery disease. The PRODIGY trial used the BARC bleeding classification system ().
Summary of Evidence Available for Bleeding Among Patients With or Without Acute Coronary Syndrome.
Among patients with ACS, Valgimigli et al.38 reported an increased risk of BARC type 2 bleeding among those randomized to 24 months of DAPT compared with six months. A significant increase was also seen for BARC type 2, 3, and 5 bleeding (). There were no differences in type 3 or type 5 bleeding (individually) or in type 3 and 5 bleeding (together).
Among patients with stable coronary artery disease, extended DAPT was associated with a significant increase in type 2 bleeding, type 2, 3, and 5 bleeding, and type 3 and 5 bleeding. There were no differences in type 3 bleeding ().
Four patients with stable coronary artery disease randomized to 24 months of DAPT experienced a fatal bleed (type 5) compared with zero patients randomized to six months of DAPT ().
Myocardial Infarction
One RCT34 reported outcome data for patients with prior acute myocardial infarction with a BMS or DES.47 Of patients with a prior myocardial infarction, 47% had STEMI and 53% had non-STEMI.
Among patients with prior myocardial infarction, outcome data were available for all-cause death, cardiovascular death, noncardiovascular death, MACCE, stent thrombosis, bleeding, and myocardial infarction.
Data were not reported separately for patients with STEMI or non-STEMI.
All-Cause Death
One RCT (DAPT34) reported all-cause death among patients with or without prior acute myocardial infarction. Among patients with prior acute myocardial infarction, there was no significant difference in the risk of all-cause death with DAPT for 12 months compared with 30 months (). Among patients with no prior myocardial infarction, there was a significantly higher risk of all-cause death among participants who received DAPT for 30 months compared with 12 months ().
Summary of Evidence Available for All-Cause Death Among Patients With or Without Prior Myocardial Infarction.
Cardiovascular Death
One RCT (DAPT34) reported cardiovascular death among patients with or without prior acute myocardial infarction.
Among patients with or without prior acute myocardial infarction, there was no significant difference in the risk of cardiovascular death with DAPT for 30 months compared with 12 months ().
Summary of Evidence Available for Cardiovascular Death Among Patients With or Without Prior Myocardial Infarction.
Noncardiovascular Death
One RCT (DAPT34) reported noncardiovascular death among patients with or without prior acute myocardial infarction ().
Summary of Evidence Available for Noncardiovascular Death Among Patients With or Without Prior Myocardial Infarction.
Among patients with prior myocardial infarction, there was no significant difference in the risk of noncardiovascular death with DAPT for 30 months compared with 12 months (). However, among patients with no prior myocardial infarction, patients who received DAPT for 30 months were at increased risk of noncardiovascular death compared with those who received DAPT for 12 months (HR 2.26; 95% CI, 1.30 to 3.94) ().
Myocardial Infarction
One RCT (DAPT34) reported myocardial infarction among patients with or without prior acute myocardial infarction. Among patients with or without prior acute myocardial infarction, the risk of myocardial infarction was significantly lower among patients who received DAPT for 30 months compared with 12 months ().
Summary of Evidence Available for Myocardial Infarction Among Patients With or Without Prior Myocardial Infarction.
Stent Thrombosis
One RCT (DAPT34) reported stent thrombosis among patients with or without prior acute myocardial infarction. Among patients with or without prior acute myocardial infarction, the risk of stent thrombosis was significantly lower among patients who received DAPT for 30 months compared with 12 months ().
Summary of Evidence Available for Stent Thrombosis Among Patients With or Without Prior Myocardial Infarction.
Major Adverse Cardiac and Cerebrovascular Events
One RCT (DAPT34) reported MACCE among patients with or without prior acute myocardial infarction ().
Summary of Evidence Available for MACCE Among Patients With or Without Prior Myocardial Infarction.
Among patients with prior acute myocardial infarction, the risk of experiencing an adverse cardiac or cerebrovascular event was significantly lower among patients who received DAPT for 30 months compared with 12 months (HR 0.56; 95% CI, 0.42 to 0.76) (). Among patients with no prior myocardial infarction, there was no statistically significant difference in the HR between the two durations of DAPT (HR 0.83; 95% CI, 0.68 to 1.02) ().
Bleeding
One RCT (DAPT34) reported bleeding among patients with or without prior acute myocardial infarction ().
Summary of Evidence Available for Bleeding Among Patients With or Without Prior Myocardial Infarction.
Among patients with prior acute myocardial infarction, the risk of a BARC type 2, 3, or 5 bleeding event was significantly higher for those who received DAPT for 30 months compared with 12 months (HR 2.14; 95% CI, 1.43 to 3.19) (). Among patients with no prior acute myocardial infarction, the risk of a BARC type 2, 3, or 5 bleeding event was significantly higher for those who received DAPT for 30 months compared with 12 months (HR 1.93; 95% CI, 1.55 to 2.42) ().
Although the data were not provided separately by bleeding type, the authors noted that fatal bleeding (type 5) was rare and not significantly different between patients with or without prior myocardial infarction (0.17% versus 0.08%, P = 0.145).47
Diabetes
In total, four RCTs (DAPT,34 ARCTIC-Interruption,35 DES-LATE,36 PRODIGY38) provided subgroup data for participants with or without diabetes.34-36,38 One RCT (DAPT34) reported death (all-cause, cardiovascular, noncardiovascular), stroke, myocardial infarction, MACCE, stent thrombosis, and major bleeding among those with or without diabetes. The remaining RCTs reported only MACCE among participants with or without diabetes.35,36,38
All-Cause Death
All-cause death was assessed among participants with and without diabetes in one RCT.34 Data were not provided separately for participants with DES or BMS. In both those with and those without diabetes, there was no significant difference in the risk of all-cause death between DAPT for 30 months compared with 12 months ().
Summary of Evidence Available for All-Cause Death Among Participants With or Without Diabetes.
Cardiovascular Death
Cardiovascular death was assessed among participants with diabetes in one RCT.34 No data were provided for participants without diabetes. Among those with diabetes and either a DES or BMS, there was no significant difference in the risk of cardiovascular death between DAPT for 30 months compared with 12 months ().
Summary of Evidence Available for Cardiovascular Death Among Participants With Diabetes.
Noncardiovascular Death
One systematic review22 reported the risk of noncardiovascular death among patients with diabetes randomized to short or extended DAPT (). Among patients with diabetes, there was no significant difference in the risk of noncardiovascular death between those who received DAPT for 12 months or 30 months (HR 1.71; 95% CI, 0.79 to 3.70).
Summary of Evidence Available for Noncardiovascular Death Among Participants With Diabetes.
Myocardial Infarction
Myocardial infarction was assessed among participants with and without diabetes in one RCT.34
Among participants with diabetes, there was no significant difference in the risk of myocardial infarction between DAPT for 30 months compared with 12 months (HR 0.72; 95% CI, 0.51 to 1.01) ().
Summary of Evidence Available for Myocardial Infarction Among Participants With or Without Diabetes.
Among participants without diabetes, DAPT for longer than 12 months was associated with a significantly lower risk of myocardial infarction compared with DAPT for 12 months (HR 0.42; 95% CI, 0.31 to 0.57) ().
Stroke
Stroke was assessed among participants with diabetes in one RCT.34 No data were provided for participants without diabetes. Among those with diabetes, there was no significant difference in the risk of stroke between DAPT for 30 months compared with DAPT for 12 months (HR 1.00; 95% CI, 0.52 to 1.95) ().
Summary of Evidence Available for Stroke Among Participants With Diabetes.
Stent Thrombosis
Stent thrombosis was assessed among participants with and without diabetes in one RCT.34 Data were available for definite, probable, and probable or definite stent thrombosis among participants with diabetes, while data were available only for probable or definite stent thrombosis among participants without diabetes ().
Summary of Evidence Available for Stent Thrombosis in Patients With or Without Diabetes.
Among participants with diabetes, there was no statistically significant difference in the risk of probable or definite stent thrombosis (HR 0.47; 95% CI, 0.21 to 1.05), probable stent thrombosis (HR 0.71; 95% CI, 0.16 to 3.17), or definite stent thrombosis (HR 0.41; 95% CI, 0.16 to 1.05) between DAPT for 30 months compared with DAPT for 12 months (). Among participants without diabetes and either a DES or BMS, DAPT for longer than 12 months was associated with a significantly lower risk of probable or definite stent thrombosis compared with DAPT for 12 months (HR 0.25; 95% CI, 0.14 to 0.45) (). Data were not provided separately for probable or definite stent thrombosis.
Major Adverse Cardiac and Cerebrovascular Events
Four RCTs (DAPT,34 ARCTIC-Interruption,35 DES-LATE,36 PRODIGY38) reported MACCE among participants with and without diabetes. Each trial used a different definition of MACCE (), making it inappropriate to combine the data via meta-analysis.
Two trials included only participants with a DES (ARCTIC-Interruption,35 DES-LATE), while two included participants with either a DES or a BMS (DAPT,34 PRODIGY38). The DAPT trial provided data separately for participants with DES34 as well as collectively for participants with either DES or BMS.48
Diabetes
Three RCTs34-36 reported no significant differences in the risk of MACCE between DAPT for six to 12 months compared with DAPT for more than 12 months among patients with diabetes with DES ().
Summary of Evidence Available for MACCE in Patients With or Without Diabetes.
Two RCTs34,38 reported no significant differences in the risk of MACCE between DAPT for six to 12 months compared with DAPT for more than 12 months among patients with diabetes and either a BMS or DES ().
When all available evidence was pooled via meta-analysis, there was no significant difference in the risk of MACCE among patients who received extended DAPT compared with six to 12 months of DAPT (risk ratio [RR] 1.12; 95% CI, 0.80 to 1.58) ().
Relative Risk of MACCE Among Patients With or Without Diabetes. CI = confidence interval; MACCE = major adverse cardiac and cerebrovascular events.
No Diabetes
Three RCTs34-36 reported there no significant differences in the risk of MACCE between DAPT for six to 12 months compared with DAPT for more than 12 months among patients without diabetes with a DES.
One RCT (PRODIGY38) reported no significant differences in the risk of MACCE between DAPT for six to 12 months compared with DAPT for more than 12 months among patients without diabetes with either a BMS or a DES.
When all available evidence was pooled via meta-analysis, there was an overall reduction in the risk of MACCE among patients who received extended DAPT compared with six to 12 months of DAPT (RR 0.66; 95% CI, 0.55 to 0.79) ().
Major Bleeding
Major bleeding was assessed among participants with diabetes in one RCT.34 The DAPT trial reported bleeding by use of the BARC system for patients with diabetes.
Among participants with diabetes, there was a statistically significant difference in the risk of type 2 and type 3 but not type 5 bleeding between DAPT for 30 months compared with 12 months (). When types 2, 3, and 5 bleeding were considered together, DAPT for 30 months was associated with an increased risk of bleeding compared with DAPT for 12 months (RR 1.60; 95% CI, 1.16 to 2.21) (). No data were provided for participants without diabetes.
Summary of Evidence Available: Bleeding in Patients With Diabetes.
Age
In total, four RCTs (DART,34 ARCTIC-Interruption,35 DES-LATE,36 PRODIGY38) provided subgroup data for participants by age. One RCT (DAPT34) reported MACCE, myocardial infarction, and stent thrombosis, while the other RCTs reported only MACCE among participants by age group.35,36,38
Myocardial Infarction
Myocardial infarction was reported by age group in one trial (DAPT34). Although the DAPT trial included participants with either a DES or a BMS, data were reported by age only for those with a DES.
Among patients aged less than 75 years, DAPT for more than 12 months was associated with a significantly lower risk of myocardial infarction (HR 0.46; 95% CI, 0.36 to 0.60) (). Among patients aged greater than 75 years, the difference in risk of myocardial infarction between 12 months and 30 months of DAPT was not statistically significant (HR 0.76; 95% CI, 0.38 to 1.54) ().
Summary of Evidence Available for Myocardial Infarction by Age Group.
Stent Thrombosis
Stent thrombosis was reported by age group in one trial (DAPT34). Although the DAPT trial included participants with either a DES or a BMS, data were reported by age only for those with a DES.
Among patients aged less than 75 years, DAPT for more than 12 months was associated with a significantly lower risk of probable or definite stent thrombosis (HR 0.29; 95% CI, 0.17 to 0.49) (). Among patients aged greater than 75 years, the difference in risk of probable or definite stent thrombosis between 12 months and 30 months of DAPT was not statistically significant (HR 0.23; 95% CI, 0.03 to 2.06) ().
Summary of Evidence Available for Stent Thrombosis by Age Group.
Major Adverse Cardiac and Cerebrovascular Events
Four RCTs (DAPT,34 ARCTIC-Interruption,35 DES-LATE,36 PRODIGY38) reported MACCE
by age group. However, the data were grouped in different ways. For example, the DES-LATE and PRODIGY trials compared participants aged less than 65 years with those aged more than 65 years. However, the ARCTIC-Interruption and DAPT trials compared participants aged less than 75 years with those aged more than 75 years. In , data are presented for younger patients and older patients according to the classification used in the RCT.
Summary of Evidence Available for MACCE by Age Group.
Although the DAPT trial included participants with either a DES or a BMS, data were reported by age only for those with a DES. Each of the included trials used a different definition of MACCE, making comparison of the findings difficult.
Among patients aged less than 75 years, three RCTs reported no statistically significant difference in the risk of MACCE. In contrast, one RCT34 reported a significantly lower risk among participants who received DAPT for 30 months compared with 12 months (HR 0.69; 95% CI, 0.57 to 0.83) ().
Among patients aged greater than 65 years, each RCT reported a non-significant difference in risk of MACCE between extended DAPT and DAPT for 12 months or less ().
Smoking
Two RCTs reported outcomes among patients described as either current tobacco users or current smokers compared with non-tobacco users or non-smokers.34,35 Both trials assessed MACCE among smokers and non-smokers, while one trial34 assessed myocardial infarction and stent thrombosis.
In the DAPT trial,34 the number of smokers and non-smokers assigned to continue DAPT (> 12 months) or to discontinue DAPT at 12 months was not reported. The total number of smokers in the trial was 2,432, while 7,529 patients reported no current tobacco use.
Myocardial Infarction
Myocardial infarction was assessed among smokers and non-smokers in the DAPT trial.34 In both smokers and non-smokers, the risk of myocardial infarction was significantly lower among patients who received DAPT for 30 months compared with 12 months ().
Summary of Evidence Available for Myocardial Infarction Among Smokers and Non-Smokers.
Stent Thrombosis
Stent thrombosis was assessed among smokers and non-smokers in the DAPT trial.34 In both smokers and non-smokers, the risk of probable or definite stent thrombosis was significantly lower among patients who received DAPT for 30 months compared with 12 months ().
Summary of Evidence Available for Stent Thrombosis Smokers Among Smokers and Non-Smokers.
Major Adverse Cardiac and Cerebrovascular Events
Two RTCs assessed MACCE among smokers and non-smokers.34,35
Among current tobacco users with a DES, Mauri et al. (DAPT34) reported a significant reduction in the risk of death, myocardial infarction, or stroke in patients who received DAPT for 18 to 30 months compared with 12 months (HR 0.66; 95% CI, 0.48 to 0.91) (). They reported a similar reduction in the risk of MACCE among non-smokers (HR 0.75; 95% CI, 0.61 to 0.93). Using a more inclusive definition of MACCE, Collet et al. (ARCTIC-Interruption35) found a non-significant difference in the odds of an event between DAPT for 12 months and DAPT for 18 to 30 months among both smokers (RR 0.83; 95% CI, 0.25 to 2.70) and non-smokers (RR 0.86; 95% CI, 0.46 to 1.59) ().
Summary of Evidence Available for MACCE Among Smokers and Non-Smokers.
Lesion Complexity
One RCT reported subgroup data by lesion complexity (PRODIGY38). Data were provided for MACCE for patients with a simple or complex lesion and an implanted DES or BMS. Complex lesions were defined as those that met the criteria for a type B2 or C lesion according to the American College of Cardiology and American Heart Association’s classification system.49,50
Among patients with a simple lesion, there was no significant difference in the risk of MACCE between participants who received extended DAPT compared with six months of DAPT (HR 1.28; 95% CI, 0.76 to 2.17) (). Similarly, among patients with a complex lesion, there was no significant different in the risk of MACCE between participants who received extended DAPT compared with six months of DAPT (HR 0.93; 95% CI, 0.67 to 1.30) ().
Summary of Evidence Available for MACCE by Lesion Complexity.
Other Concurrent Disease: Peripheral Artery Disease
One trial reported outcomes among patients with peripheral artery disease (PRODIGY38). Of the patients randomized in the PRODIGY trial, 12.5% had a history of peripheral artery disease.51
Outcome data were reported for all-cause death, cardiovascular death, myocardial infarction, stent thrombosis, and MACCE.
All-Cause Death
All-cause death was assessed among patients with or without peripheral artery disease in the PRODIGY trial.34
Among participants with peripheral artery disease, the risk of all-cause death was significantly lower among patients who received DAPT for 24 months compared with six months (HR 0.45; 95% CI, 0.23 to 0.88) (). Among participants without peripheral artery disease, there was no significant difference in the risk of all-cause death between DAPT for 24 months compared with six months (HR 1.39; 95% CI, 0.91 to 2.10) ().
Summary of Evidence Available for All-Cause Death Among Patients With or Without Peripheral Artery Disease.
Cardiovascular Death
Cardiovascular death was assessed among patients with or without peripheral artery disease in the PRODIGY trial.34
Among participants with peripheral artery disease, the risk of cardiovascular death was significantly lower among patients who received DAPT for 24 months compared with six months (HR 0.42; 95% CI, 0.17 to 1.00) (). Among participants without peripheral artery disease, there was no significant difference in the risk of all-cause death between DAPT for 24 months compared with six months (HR 1.44; 95% CI, 0.81 to 2.55) ().
Summary of Evidence Available for Cardiovascular Death Among Patients With or Without Peripheral Artery Disease.
Myocardial Infarction
Myocardial infarction was assessed among patients with or without peripheral artery disease in the PRODIGY trial.34
Among participants with peripheral artery disease, there was no significant difference in the risk of myocardial infarction between patients who received DAPT for 24 months compared with six months (HR 0.68; 95% CI, 0.26 to 1.74) (). Among participants without peripheral artery disease, there was no significant difference in the risk of myocardial infarction between DAPT for 24 months compared with six months (HR 1.05; 95% CI, 0.64 to 1.73) ().
Summary of Evidence Available for Myocardial Infarction Among Patients With or Without Peripheral Artery Disease.
Stent Thrombosis
Stent thrombosis was assessed among patients with or without peripheral artery disease in the PRODIGY trial.34
Among participants either with or without peripheral artery disease, there was no significant difference in the risk of definite stent thrombosis between patients who received DAPT for 24 months compared with six months ().
Summary of Evidence Available for Stent Thrombosis Among Patients With or Without Peripheral Artery Disease.
Among participants with or without peripheral artery disease, there was no significant difference in the risk of definite or probable stent thrombosis between DAPT for 24 months compared with six months ().
Major Adverse Cardiac and Cerebrovascular Events
MACCE was assessed among patients with or without peripheral artery disease in the PRODIGY trial.34
Among participants with peripheral artery disease, there was a significantly lower risk of MACCE among patients who received DAPT for 24 months compared with six months (HR 0.54; 95% CI, 0.31 to 0.95) (). Among participants without peripheral artery disease, there was no significant difference in the risk of myocardial infarction between DAPT for 24 months compared with six months (HR 1.28; 95% CI, 0.92 to 1.77) ().
Summary of Evidence Available for MACCE Among Patients With or Without Peripheral Artery Disease.
History of Heart Failure
None of the included studies reported data for subgroups of patients with a history of heart failure.
Unstable Angina
None of the included studies reported data for subgroups of patients with a history of unstable angina.
Vein Graft Intervention
None of the included studies reported data for subgroups of patients with a history of vein graft intervention.
Left Main Intervention
None of the included RCTs reported data separately for patients who had undergone left main intervention.
Two RCTs reported data for patients with either left main disease (DES-LATE36) or left main or proximal left anterior descending coronary artery disease (PRODIGY38). Of the patients with left main disease in the DES-LATE trial, 64% underwent left main intervention,36 while 7% of patients with lumen narrowing of the left main or proximal left coronary artery underwent stenting of the left main artery in the PRODIGY trial.38 Thus, no data were available to address the question of the optimal duration of DAPT among patients who had undergone left main intervention.
Data Summary: Research Question 3
Does the optimal duration of DAPT with ASA plus a P2Y12 inhibitor (clopidogrel, prasugrel, ticagrelor) depend on the type of stent implanted (BMS or DES)?
Each of the included systematic reviews involved RCTs that enrolled patients with a DES. Two RCTs also involved patients with a BMS (DAPT34, PRODIGY38)
To address this research question, data were extracted from systematic reviews that assessed outcomes only among participants with a DES. None of the included systematic reviews reported outcomes among patients with a BMS. Thus, we sought out data from RCTs included in the systematic reviews to address this research question.
Drug-Eluting Stents
All-Cause Death
Six Months Versus More Than 12 Months
Two systematic reviews assessed all-cause death among patients with a DES.16,22
Among patients with any type of DES, Palmerini et al.22 reported no significant difference in the risk of all-cause death between six months and 24 months of DAPT (HR 1.10; 95% CI, 0.73 to 1.63) (). Among patients with a second-generation DES, D’Ascenzo et al.16 reported no significant difference in the odds of all-cause death between six months and 24 months of DAPT (OR 1.11; 95% CI, 0.35 to 3.57) ().
Summary of Evidence Available for All-Cause Death Among Patients With a Drug-Eluting Stent: 6 Months Versus > 12 Months.
One systematic review29 compared all durations of DAPT via network meta-analysis involving patients who had undergone PCI with a DES. In their analysis, Xie et al.29 found no significant difference in the odds of all-cause death between six months of DAPT and 24, 30, or 36 months of DAPT ().
Summary of Evidence Available for All-Cause Death Among Patients With a Drug-Eluting Stent: Network Meta-Analysis, 6 Months Versus > 12 Months.
Six to 12 Months Versus More Than 12 Months
One review33 assessed all-cause death among patients with a DES randomized to six to 12 months or more than 12 months of DAPT. Bittl et al.33 reported no significant difference in the odds of all-cause death between those who received six to 12 months or more than 12 months of DAPT (OR 1.14; 95% CI, 0.92 to 1.42) ().
Summary of Evidence Available for All-Cause Death Among Patients With a Drug-Eluting Stent: 6 to 12 Months Versus > 12 Months.
Twelve Months Versus More Than 12 Months
Six systematic reviews13,14,16,17,22,23,30 assessed all-cause death among patients with any DES who received 12 months or more than 12 months of DAPT, while one review assessed death among those with a second-generation DES.16
Among patients who received any type of DES, six reviews reported an increased odds or risk of all-cause death among patients receiving DAPT for more than 12 months compared with those who received DAPT for 12 months (). Among patients with a secondgeneration DES, one review16 reported no statistically significant difference in all-cause death between 12 months and more than 12 months of DAPT (OR 1.67; 95% CI, 0.76 to 3.57) ().
Summary of Evidence Available for All-Cause Death Among Patients With a Drug-Eluting Stent: 12 Months Versus > 12 Months, DES.
Among patients with any type of DES, Xie et al.29 found no significant difference in the odds of all-cause death between 12 months and 24, 30, or 36 months of DAPT ().
Summary of Evidence Available for All-Cause Death Among Patients With a Drug-Eluting Stent: Network Meta-Analysis, 12 Months Versus > 12 Months.
> 12 Months Versus More Than 12 Months
Among patients with any type of DES, Xie et al.29 reported no significant difference in the odds of all-cause death between 24 months and 30 or 36 months of DAPT or between 30 months and 36 months of DAPT ().
Summary of Evidence Available for All-Cause Death Among Patients With a Drug-Eluting Stent: Network Meta-Analysis, > 12 Months.
Cardiovascular Death
Six Months Versus More Than 12 Months
In two systematic reviews, cardiovascular death among patients with a DES who received six months of DAPT was compared with patients receiving more than 12 months of DAPT.16,22 Both included only one RCT for this time comparison (PRODIGY38), with a duration of six months compared with 24 months. Although some patients in the PRODIGY trial had an implanted BMS, these patients were excluded from the analysis.
Among patients with any type of DES, Palmerini et al.22 found no significant difference in the risk of cardiovascular death between six months and greater than 12 months of DAPT (HR 1.09; 95% CI, 0.63 to 1.89) ().
Summary of Evidence Available: Cardiovascular Death Among Patients With a Drug-Eluting Stent: 6 Months Versus > 12 Months.
Among patients with a second-generation DES, D’Ascenzo et al.16 found no significant difference in the odds of cardiovascular death between six months of DAPT and 24 months (OR 0.60; 95% CI, 0.24 to 1.49) ().
Twelve Versus More Than 12 Months
Seven systematic reviews assessed cardiovascular death among patients with a DES who received 12 months of DAPT compared with those who received more than 12 months of DAPT.13,14,16,17,22,23,30
Among patients with any type of DES, each review reported no significant difference in the odds or risk of cardiovascular death between 12 months and more than 12 months of DAPT ().
Summary of Evidence Available for Cardiovascular Death Among Patients With a Drug-Eluting Stent: 12 Months Versus > 12 Months.
Among patients with a second-generation DES, D’Ascenzo et al.16 reported no significant difference in the odds of cardiovascular death between 12 months and more than 12 months of DAPT (OR 1.61; 95% CI, 0.28 to 9.09) ().
Noncardiovascular Death
One systematic review22 reported the risk of noncardiovascular death among patients with a DES randomized to short DAPT or extended DAPT ().
Summary of Evidence Available for Noncardiovascular Death Among Patients With a Drug-Eluting Stent.
Among patients with an implanted DES, there was no significant difference in the risk of noncardiovascular death between those who received DAPT for six months or more than 12 months ().
In contrast, there was a significant increase in the risk of noncardiovascular death among those who received DAPT for more than 12 months compared with those who received DAPT for 12 months (HR 1.89; 95% CI, 1.27 to 2.78) ().
Myocardial Infarction
Six Months Versus More Than 12 Months
Two systematic reviews16,29 assessed the risk of myocardial infarction among patients with a DES.
Among patients with a second-generation DES, there was no significant difference in the odds of myocardial infarction between six months and 24 months of DAPT (OR 1.23; 95% CI, 0.21 to 7.69) ().
Summary of Evidence Available for Myocardial Infarction Among Patients With a Drug-Eluting Stent: 6 Months Versus > 12 Months.
Among patients with any generation of DES, Xie et al.29 found no significant difference in the odds of myocardial infarction between six months of DAPT and 24, 30, or 36 months of DAPT ().
Summary of Evidence Available for Myocardial Infarction Among Patients With a Drug-Eluting Stent: Network Meta-Analysis.
Six to 12 Months Versus More Than 12 Months
One systematic review assessed myocardial infarction among patients who received DAPT for six to 12 months compared with more than 12 months.
Bittl et al.33 reported that the odds of myocardial infarction were significantly lower among patients with a DES who received DAPT for at least 12 months compared with patients who received DAPT for six to 12 months (OR 0.67; 95% CI, 0.47 to 0.65) ().
Summary of Evidence Available for Myocardial Infarction Among Patients With a Drug-Eluting Stent: 6 to 12 Months Versus > 12 Months.
Twelve Months Versus More Than 12 Months
Six systematic reviews13,14,16,17,23,30 assessed myocardial infarction among patients with a DES who received DAPT for 12 months compared with more than 12 months. Five included patients with any type of DES,13,14,17,23,30 while one included only participants with a secondgeneration DES.16
Among patients with any type of DES, all of the included reviews reported that the odds of myocardial infarction were significantly lower among patients who received DAPT for more than 12 months compared with patients who received DAPT for 12 months (). The findings were consistent among patients with a second-generation DES: The odds or risk of myocardial infarction were significantly lower among patients who received more than 12 months of DAPT compared with those who received 12 months of DAPT (OR 0.62; 95% CI, 0.45 to 0.86) ().
Summary of Evidence Available for Myocardial Infarction Among Patients With a Drug-Eluting Stent: 12 Months Versus > 12 Months.
Via network meta-analysis, Xie et al.29 found no significant differences in the odds of myocardial infarction between 24 months and 30 or 36 months or between 30 months and 36 months among patients with any type of DES ().
Summary of Evidence Available for Myocardial Infarction Among Patients With a Drug-Eluting Stent: Network Meta-Analysis, > 12 Months.
Stroke
Six Months Versus More Than 12 Months
None of the included systematic reviews assessed stroke among patients with a DES who received DAPT for six months compared with more than 12 months.
Six to 12 Months Versus More Than 12 Months
None of the included systematic reviews assessed stroke among patients with a DES who received DAPT for six to 12 months compared with more than 12 months.
Twelve Months Versus More Than 12 Months
Four systematic reviews13,14,17,30 assessed stroke among patients with a DES who received DAPT for 12 months compared with more than 12 months.
Among patients with any type of DES, each review reported no statistically significant difference in the odds or risk of stroke between patients who received DAPT for 12 months and those who received DAPT for more than 12 months ().
Summary of Evidence Available for Stroke Among Patients With a Drug-Eluting Stent: 12 Months Versus > 12 Months.
Stent Thrombosis
Six Months Versus More Than 12 Months
One systematic review16 assessed stent thrombosis among patients with a DES who received DAPT for six months compared with more than 12 months. Definite and probable stent thrombosis events were combined in this analysis.
Among patients with a second-generation DES, D’Ascenzo et al.16 reported no significant difference in the odds of stent thrombosis between six months of DAPT and more than 12 months of DAPT (OR 2.50; 95% CI, 0.33 to 20.00) ().
Summary of Evidence Available for Stent Thrombosis Among Patients With a Drug-Eluting Stent: 6 Months Versus > 12 Months.
Among patients with any type of DES, Xie et al.29 reported no statistically significant difference in the odds of stent thrombosis between six months and 24 months or 30 months. However, the authors reported that 30 months of DAPT was associated with statistically lower odds of stent thrombosis compared with six months of DAPT in this population (OR 0.09; 95% CI, 0.11 to 0.62) ().
Summary of Evidence Available for Stent Thrombosis Among Patients With a Drug-Eluting Stent: Network Meta-Analysis, 6 Months Versus > 12 Months.
Six to 12 Months Versus More Than 12 Months
One systematic review33 assessed stent thrombosis among patients who received DAPT for six to 12 months compared with more than 12 months.
Among patients with any type of DES, Bittl et al.33 reported that the odds of stent thrombosis were significantly lower among patients who received DAPT for more than 12 months compared with patients who received DAPT for six to 12 months (OR 0.42; 95% CI, 0.24 to 0.74) ().
Summary of Evidence Available for Stent Thrombosis Among Patients With a Drug-Eluting Stent: 6 to 12 Months Versus > 12 Months.
Twelve Months Versus More Than 12 Months
Six systematic reviews assessed stent thrombosis among patients with a DES who received DAPT for 12 months compared with more than 12 months.13,14,16,17,23,30 Each review assessed definite/probable stent thrombosis; as well, Navarese et al.23 assessed definite stent thrombosis and very late stent thrombosis (i.e., stent thrombosis occurring more than one year after PCI) ().
Summary of Evidence Available for Stent Thrombosis Among Patients With a Drug-Eluting Stent: 12 Months Versus > 12 Months.
Five reviews13,14,17,23,30 that included any type of DES reported that the odds or risk of probable or definite stent thrombosis were significantly lower among patients who received DAPT for more than 12 months compared with patients who received DAPT for 12 months ().
One review that included only second-generation DES (everolimus-eluting stent or zotarolimus-eluting stent) reported no statistically significant difference in probable or definite stent thrombosis between 12 months of DAPT and more than 12 months of DAPT (OR 0.54; 95% CI, 0.87 to 1.02) ().
One review23 reported that more than 12 months of DAPT was associated with significantly lower odds of definite stent thrombosis compared with 12 months of DAPT in patients with any type of DES (OR 0.30; 95% CI, 0.19 to 0.49) (). Navarese et al.23 also reported significantly lower odds of very late stent thrombosis among patients who received more than 12 months of DAPT in this population (OR 0.33; 95% CI, 0.21 to 0.51).
Among patients with a DES, Xie et al.29 reported no statistically significant difference in the odds of stent thrombosis between 12 months and 24 or 36 months of DAPT (). The authors reported that 30 months of DAPT was associated with statistically lower odds of stent thrombosis compared with 12 months of DAPT in this population (OR 0.25; 95% CI, 0.09 to 0.99) ().
Summary of Evidence Available for Stent Thrombosis Among Patients With a Drug-Eluting Stent: Network Meta-Analysis, 12 Months Versus > 12 Months.
Longer Than 12 Months
Among patients with any type of DES, Xie et al.29 found no statistically significant difference in the odds of stent thrombosis between any durations longer than 12 months ().
Summary of Evidence Available for Stent Thrombosis Among Patients With a Drug-Eluting Stent: Network Meta-Analysis, > 12 Months.
Urgent Target Revascularization
None of the included systematic reviews assessed urgent revascularization. This outcome was reported in two of the included RCTs (ITALIC,37 ARCTIC-Interruption35); findings are summarized in . There were no significant differences in risk between short DAPT and extended DAPT in either trial.
Summary of Evidence Available for Urgent Target Revascularization.
Major Adverse Cardiovascular Events
Two systematic reviews assessed major adverse cardiovascular events associated with different durations of DAPT among patients with a DES.23,33 One review each compared six to 12 months of DAPT with more than 12 months33 of DAPT and 12 months of DAPT with more than 12 months of DAPT.23 Bittl et al.33 described this outcome as a comparison of the primary outcomes of each RCT ().
Summary of Evidence Available for MACCE Among Patients With a Drug-Eluting Stent.
Despite differences in the outcome definition, both Bittl et al.33 and Navarese et al.23 reported significantly lower odds of an event among patients with a DES who received more than 12 months of DAPT ().
Major Bleeding
Two systematic reviews assessed major bleeding among patients with a DES.16,23
Among patients with any type of DES, Navarese et al.23 reported no statistically significant increase in the odds of TIMI major bleeding with extended DAPT (OR 1.60; 95% CI, 0.97 to 2.64) (). Among patients with a second-generation DES, D’Ascenzo et al.16 found an increased odds of BARC type 3 or 5 bleeding associated with DAPT for 24 months compared with DAPT of six months (OR 1.82; 95% CI, 1.02 to 3.23) ().
Summary of Evidence Available for Major Bleeding Among Patients With a Drug-Eluting Stent.
Minor Bleeding
None of the included systematic reviews assessed minor bleeding in patients with a drugeluting stent.
Gastrointestinal Bleeding
None of the included systematic reviews assessed gastrointestinal bleeding among patients with a DES.
Bare-Metal Stents
None of the included systematic reviews reported outcomes among participants with a BMS. As such, data were extracted from the two RCTs that involved participants with a BMS as well as patients with a DES (DAPT,34 PRODIGY38). Both RCTs supplied subgroup analyses for patients only with a BMS; however, not all outcomes were reported.
All-Cause Death
Two RCTs (DAPT,34 PRODIGY38) reported all-cause death among participants with a BMS ().
Summary of Evidence Available: All-Cause Death Among Patients With a Bare-Metal Stent.
Mauri et al.34 reported that there was no significant difference in the risk of all-cause death between 12 months and 30 months of DAPT (HR 0.90; 95% CI, 0.35 to 2.33) (). The risk of all-cause death was also not significantly different between six months and 24 months of DAPT in the PRODIGY trial.38
The pooled data from the DAPT34 and PRODIGY38 trials supports no significant difference in the risk of all-cause death between extended DAPT and six to 12 months of DAPT among patients with a BMS (RR 0.80; 95% CI, 0.48 to 1.35) ().
Relative Risk of All-Cause Death Among Patients With a Bare-Metal Stent. CI = confidence interval; mo = months.
Cardiovascular Death
One RCT (PRODIGY38) reported cardiovascular death among participants with a BMS.
Calculated based on reported event counts, the risk of cardiovascular death was not significantly different between six months and 24 months of DAPT in the PRODIGY trial ().38
Summary of Evidence Available: Cardiovascular Death Among Patients With a Bare-Metal Stent.
Myocardial Infarction
Two RCTs (DAPT,34 PRODIGY38) reported myocardial infarction among participants with a BMS.
Mauri et al.34 reported no significant differences in the risk of myocardial infarction between 12 months and 30 months of DAPT (HR 0.91; 95% CI, 0.51 to 1.62) (). The risk of myocardial infarction was also not significantly different between six months of DAPT and 24 months of DAPT in the PRODIGY trial (RR 1.00; 95% CI, 0.50 to 2.01) ().38
Summary of Evidence Available for Myocardial Infarction Among Patients With a Bare-Metal Stent.
The pooled data from the DAPT34 and PRODIGY38 trials supports no significant difference in the risk of myocardial infarction between extended DAPT and six to 12 months of DAPT among patients with a BMS (RR 0.90; 95% CI, 0.58 to 1.41) ().
Relative Risk of Myocardial Infarction Among Patients With a Bare-Metal Stent. CI = confidence interval; mo = months.
Stroke
Two RCTs (DAPT,34 PRODIGY38) reported stroke among study participants with a BMS.
Mauri et al.34 reported that there was no significant difference in the risk of stroke between 12 months and 30 months of DAPT (HR 1.22; 95% CI, 0.37 to 4.01) (). Calculated based on reported event counts, the risk of stroke was also not significantly different between six months and 24 months of DAPT in the PRODIGY trial (RR 1.41; 95% CI, 0.45 to 4.37) ().38
Summary of Evidence Available for Stroke Among Patients With a Bare-Metal Stent.
The pooled data from the DAPT34 and PRODIGY38 trials supports no significant difference in the risk of stroke between extended DAPT and six to 12 months of DAPT among patients with a BMS (RR 1.31; 95% CI, 0.58 to 2.96) ().
Relative Risk of Stroke Among Patients With a Bare-Metal Stent. CI = confidence interval; mo = months.
Stent Thrombosis
Two RCTs (DAPT,34 PRODIGY38) reported stent thrombosis among participants with a BMS.
Mauri et al.34 reported that there was no significant difference in the risk of stent thrombosis between 12 months and 30 months of DAPT (HR 0.49; 95% CI 0.15 to 1.64) (). The timing of stent thrombosis (late or very late) was not reported. The risk of definite or probable late stent thrombosis was not significantly different between six months and 24 months of DAPT in the PRODIGY trial (RR 1.00; 95% CI, 0.29 to 3.42) ().38 Similarly, the risk of definite late stent thrombosis was not significantly different between six months of DAPT and 24 months of DAPT in the PRODIGY trial (RR 3.01; 95% CI, 0.32 to 28.76).38
Summary of Evidence Available for Stent Thrombosis Among Patients With a Bare-Metal Stent.
Major Adverse Cardiac and Cerebrovascular Events
Two RCTs (DAPT,34 PRODIGY38) reported MACCE among participants with a BMS.
Despite differences in the definition of MACCE in each trial, both the DAPT34 and PRODIGY38 trials reported non-significant differences in the risk of MACCE between DAPT for six to 12 months and DAPT for more than 12 months ().
Summary of Evidence Available for MACCE Among Patients With a Bare-Metal Stent.
The pooled data from the DAPT34 and PRODIGY38 trials supports no significant difference in the risk of MACCE between DAPT for more than 12 months compared with DAPT for six to 12 months among patients with a BMS (RR 0.89; 95% CI, 0.64 to 1.23) ().
Relative Risk of MACCE Among Patients With a Bare-Metal Stent. CI = confidence interval. mo = months.
Major Bleeding
One RCT (DAPT34) reported major bleeding among participants with a BMS.
Participants who received DAPT for 30 months were at higher risk of BARC type 2 bleeding (risk difference 1.88%; 95% CI, 0.56% to 3.21%) and BARC type 3 bleeding (risk difference 1.25%; 95% CI, 0.09% to 2.41%) compared with participants who received DAPT for 12 months ().
Summary of Evidence Available for Major Bleeding Among Patients With a Bare-Metal Stent.
There was no significant difference in the risk of fatal bleeding (type 5) between DAPT for 12 months or for 30 months (risk difference –0.13%; 95% CI. –0.38% to 0.12%) ().
When considered together, the risk of type 2, 3, or 5 bleeding was significantly higher among patients who received DAPT for 30 months compared with 12 months (risk difference 2.75%; 95% CI, 1.02% to 4.48%) ().
Minor Bleeding
No data were reported for minor bleeding among patients with a BMS.
Gastrointestinal Bleeding
No data were reported for gastrointestinal bleeding among patients with a BMS.
Data Summary: Research Question 4
Is there a rebound effect after withdrawal of P2Y12 inhibitor (clopidogrel, prasugrel, ticagrelor) therapy?
None of the included systematic reviews assessed an outcome of interest after withdrawal of DAPT.
Of the RCTs included in the systematic reviews, only one trial (DAPT34) reported outcomes after the discontinuation of DAPT. Data were available for patients with any type of DES from one RCT (DAPT34) for all-cause death, stent thrombosis, myocardial infarction, stroke, MACCE, and major bleeding.
The authors of the DAPT trial qualitatively assessed the hazards before and after discontinuation of DAPT following 12 months or 30 months of therapy, and concluded that there was an elevated risk of stent thrombosis and myocardial infarction in the three months following discontinuation of DAPT.
In an inferential analysis, we performed a large sample test to assess for quantitative differences in hazard rate for each outcome before and after discontinuation of DAPT ().
Summary of Evidence Before and After Withdrawal of DAPT Among Patients With a Drug-Eluting Stent.
Among patients who received 12 months of DAPT, there was no significant difference in the risk of all-cause death, myocardial infarction, stent thrombosis, or MACCE after discontinuation of DAPT ().
Among patients who received DAPT for 30 months, the risk of myocardial infarction and MACCE was higher in the first three months after discontinuation compared with the last three months before discontinuation. There was no significant difference in the risk of allcause death, stent thrombosis, or stroke after discontinuation of DAPT ().
Patients With Diabetes
One RCT (DAPT34) assessed outcomes after discontinuation of DAPT among participants with diabetes with an implanted DES or BMS.
Myocardial infarction: After receiving DAPT for 30 months, in the subsequent three-month period with no DAPT (months 30 to 33), 1.1% of participants with diabetes experienced a myocardial infarction. During the same period, 0.6% of participants who had received only 12 months of DAPT experienced a myocardial infarction (non-significant difference between groups, P = 0.12).
Stent thrombosis: After receiving DAPT for 30 months, in the subsequent three-month period with no DAPT (months 30 to 33), 0.3% of participants with diabetes experienced stent thrombosis. During the same period, 0.09% of participants who had received only 12 months of DAPT experienced a stent thrombosis (non-significant difference between groups, P = 0.11).
MACCE: After receiving DAPT for 30 months, in the subsequent three-month period with no DAPT (months 30 to 33), 2.1% of participants with diabetes experienced an event. During the same period, 1.2% of participants who had received only 12 months of DAPT experienced a stent thrombosis (significant difference between groups, P = 0.04).