Summary
Within the past 15 years, tremendous advances in diabetes care have occurred, including increases in the number of medication classes approved to treat type 2 diabetes and technologies to help manage type 1 and type 2 diabetes. In addition, many of the newer classes of medications have been used more extensively given their efficacy in terms of glycemia, weight loss, and cardiovascular and renal protection. None of these new agents have been approved for the treatment of type 1 diabetes, yet they are occasionally used off-label. This article reviews diabetes treatment and self-care practices for adults and youth with type 1 and type 2 diabetes in the U.S. population.
Among insured adults with type 1 diabetes whose claims are included in the 2019 Optum Clinformatics Data Mart database, insulin is used as the single antihyperglycemic agent 88% of the time, with the remaining 12% of individuals using insulin plus another agent, most often metformin. In youth with type 1 diabetes, insulin remains the mainstay treatment. Use of continuous glucose monitors (CGMs) has become a common mode of glucose monitoring for both adults and youth with type 1 diabetes.
From a public health perspective, diabetes technology, especially CGM, is underutilized by persons with type 1 diabetes, perhaps even more so in adults than children. For example, only 41% of adults with type 1 diabetes used CGM in 2020. Inequities in technology usage persist. Increased use of insulin pumps, CGM, sensor-augmented pumps, and automated insulin delivery is associated with a greater proportion of individuals achieving target glycated hemoglobin (A1C) in both adults and children with type 1 diabetes.
For adults with type 2 diabetes, the most common antihyperglycemic drug class used was biguanide (i.e., metformin), followed by sulfonylurea and insulin, whereas thiazolidinedione was the least used medication in 2015–2020. In adults with type 2 diabetes, increased use of the GLP-1 receptor agonists (GLP-1 RAs) and sodium-glucose cotransporter-2 (SGLT2) inhibitors, when appropriate, could have a beneficial impact on cardiovascular and renal disease. Use of CGM has become more common among adults with type 2 diabetes and is now covered by most private insurance and by the Centers for Medicare and Medicaid Services (CMS). The cost of and access to these newer technologies and medications have been major barriers.
Type 2 diabetes has become increasingly more common among youth over the past two decades, and compared to type 2 diabetes diagnosed in adults, it is a more aggressive disease and less responsive to metformin. Biguanides and insulin are antihyperglycemic medications approved for youth with type 2 diabetes in conjunction with lifestyle modifications. Very few data are available on technology use among youth with type 2 diabetes.
Introduction
Diabetes mellitus is a complex condition requiring great attention to lifestyle, as well as medication use and glucose monitoring often multiple times a day. Medications used to treat diabetes include insulin and other antihyperglycemic medications, as well as nonglycemic medications used to treat comorbidities. The number of antihyperglycemic medications that are available to treat diabetes continues to grow, and advances in technology for glucose monitoring and insulin administration have changed the landscape of diabetes care. Thus, the content herein updates data published in the Medication Use and Self-Care Practices in Persons With Diabetes chapter of Diabetes in America, 3rd edition (1) on the use of newer classes of medications and improved technologies that are being used more extensively by adults and youth with diabetes.
Trends in current medication and technology use and self-care practices among adults and youth with type 1 and type 2 diabetes in the United States are described. Data for each of the four populations (adults with type 1 diabetes, youth with type 1 diabetes, adults with type 2 diabetes, and youth with type 2 diabetes) are provided from different data sources, as the availability of population-level data varies with each subgroup. Data on adults with type 1 diabetes come from Optum Clinformatics Data Mart (2) and the IBM MarketScan commercial database (3,4); these claim-based data sources are not nationally representative of the U.S. population as they only include persons with commercial health insurance. Data on youth with type 1 diabetes are from the SEARCH for Diabetes in Youth study (SEARCH) (5,6,7) and the T1D Exchange (8). Data on adults with type 2 diabetes are from the National Health and Nutrition Examination Survey (NHANES) (9), the National Health Interview Survey (NHIS) (10), and Centricity Electronic Medical Records (11). Data on youth with type 2 diabetes are from SEARCH (5) and the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study (12).
Sources and Limitations of National Data on Medication Use and Self-Care Practices and Diabetes
Information on medication use and self-care practices in individuals with diagnosed diabetes is available from several surveys conducted in the United States that use national probability samples, including the NHANES (9) and NHIS (10). The Type 2 Diabetes in Adults section utilizes data from these sources.
The NHANES is a cross-sectional, national probability sample that has been conducted periodically since 1971 and continuously since 1999. NHANES data collected from individuals for the period 2015–March 2020 were utilized to assess medication use and self-care practices among adults with diagnosed type 2 diabetes. Participants self-reported diabetes, based on the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Since medication use differs by type of diabetes, individuals with probable type 1 diabetes, defined as insulin use only and continuous insulin use within 1 year of diagnosis (13), were excluded from analysis. Analysis among adults with probable type 1 diabetes is not feasible in the NHANES due to the small sample size. An advantage of the NHANES is that the survey includes a health exam in a mobile examination center where laboratory measures are collected. Thus, the NHANES has laboratory information on glycated hemoglobin (A1C) and fasting plasma glucose (FPG) to determine undiagnosed diabetes. Participants self-reported sociodemographic characteristics, prescription medication use in the past 30 days, and self-care practices. Participants were asked to bring their prescription medication bottles to the interview for verification by the interviewer.
The NHIS is a cross-sectional household interview survey that uses a complex sampling design and has been conducted continuously since 1957. NHIS 2019 survey data were used to assess low-dose aspirin therapy among adults with diagnosed type 2 diabetes. In the NHIS, diabetes is defined by participants responding “yes” to the following question: “(If female, other than during pregnancy) Have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Probable type 1 diabetes is defined as insulin use only and continuous insulin use within 1 year of diagnosis. Questions about aspirin use include whether low-dose aspirin was medically advised and whether low-dose aspirin is currently being used. Although the questionnaire items in the NHIS are comprehensive, a limitation is that the data are self-reported.
Statistical Methods
All estimates from national surveys are weighted to produce estimates that are nationally representative of the noninstitutionalized U.S. population. This article provides weighted standard errors for estimates in tables. The relative standard error (RSE = [SE/estimate]*100) is provided in tables and figures for estimates that are likely unreliable due to sample size. Estimates with RSEs >50% are censored.
Type 1 Diabetes in Adults
Overview
Nationally representative estimates from the NHIS 2019–2021 suggest that 1.7 million U.S. adults age ≥20 years self-reported having type 1 diabetes and using insulin, which roughly estimates the prevalence of type 1 diabetes in the U.S. adult population (14). This represents 5.7% of all U.S. adults with diagnosed diabetes.
An analysis of the 2019 Optum Clinformatics Data Mart was conducted to assess the use of antihyperglycemic and nonglycemic medications among adults with type 1 diabetes. Adults with type 1 diabetes were defined as having at least one type 1 diabetes International Classification of Diseases, Tenth Revision (ICD10) code (E10), no type 2 diabetes ICD10 code (E11), and an insulin prescription. Data on diabetes technology among adults with type 1 diabetes (defined as in Optum, using ICD10 code E10 and an insulin prescription) are presented from a published study that utilized the IBM MarketScan commercial database (15). Both databases utilize electronic medical records and administrative billing claims. The main weakness of the Optum and MarketScan databases is that they only include persons with commercial insurance, including Medicare Advantage. Persons insured through Medicare or Medicaid and persons who are uninsured are not represented in these data. Additionally, the IBM MarketScan commercial database does not include individuals age ≥65 years. All medication data reported for adults with type 1 diabetes derive from this population.
Antihyperglycemic Medications
The only medications approved to treat type 1 diabetes include injectable or inhaled formulations of insulin and pramlintide, an amylin analogue (16). Indeed, daily insulin is required for survival. Studies have shown mixed results for glycemic efficacy and safety for antihyperglycemic medications only approved for type 2 diabetes (16,17). To date, no primary cardiovascular, renal, or obesity trials using these off-label medications have been conducted among adults with type 1 diabetes.
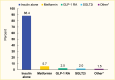
Based on the 2019 Optum Clinformatics Data Mart, 11.6% of commercially insured patients with type 1 diabetes were taking a non-insulin antihyperglycemic medication in addition to insulin, while 88.4% were taking insulin only (Figure 1). Metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter-2 (SGLT2) inhibitors, and other agents were used by 5.7%, 2.5%, 2.0%, and 1.5% of patients, respectively. In this analysis, amylin analogues, alpha-glucosidase inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors, thiazolidinediones, sulfonylureas, and meglitinides are classified as “other” medications.
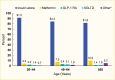
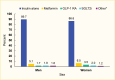
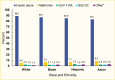
Medication use was similar by age, although metformin use tended to increase with age (4.2% and 10.0% in the 20–44 years and ≥65 years age groups, respectively) (Figure 2). Few differences were seen between men and women, although women were more likely to use GLP-1 RAs (3.5% in women vs. 1.7% in men) (Figure 3). Eighty-four percent (83.7%) of this commercially insured sample of adults with type 1 diabetes were White, while 7.0% were Black, 7.1% Hispanic, and 2.1% Asian. In general, White individuals were less likely to use non-insulin medications compared to individuals from other racial and ethnic groups (Figure 4).
Medications for Other Health Conditions
Despite longer life expectancy than in the past (particularly in high-income countries such as the United States), adults with type 1 diabetes have a greater and earlier risk of both kidney disease and premature cardiovascular disease than those without diabetes (18,19). Thus, adults with type 1 diabetes often utilize nonglycemic medications (i.e., antihypertensive, lipid-lowering, etc.) to treat diabetes-related comorbidities and complications. By 50 years of diabetes duration, well over half of the population with type 1 diabetes have significant renal disease (20). In type 1 diabetes complicated by kidney disease, inhibition of the renin-angiotensin-aldosterone system provides more benefit than blood pressure control alone (21). Although studies are sparse, the data also suggest those with type 1 diabetes have a 30% higher cardiovascular disease mortality compared to those without diabetes (22). In a Swedish registry with almost 34,000 people with type 1 diabetes, the adjusted hazard ratio for cardiovascular death compared to controls without diabetes was 4.60 (95% CI 3.47–6.10) (19). Unfortunately, no prospective trials have addressed blood pressure or lipid control for cardiovascular endpoints, yet hypertension and low-density lipoprotein (LDL) targets are the same for persons with either type 1 or type 2 diabetes (23). An observational study reported that lipid-lowering therapy was associated with a 22%–44% reduction in the risk of cardiovascular disease and death among individuals with type 1 diabetes without a prior history of cardiovascular disease (24).
Another common comorbidity of diabetes is depression, which is three times more common in adults with type 1 diabetes relative to adults without diabetes. Thus, the use of antidepressants is often more common in this population (25).
In 2019, adults with type 1 diabetes averaged 2.7 nonglycemic medications, and this number increased with age (Table 1, Appendix Table A1). Almost 41% were receiving antihypertensives, most commonly angiotensin-converting enzyme (ACE) inhibitors, followed by beta blockers and angiotensin II receptor blockers (ARBs). For lipid-lowering medications, 35.2% were receiving statins, but that number increased to 72.0% for individuals age ≥65 years. Among all adults with type 1 diabetes, 20.6% were receiving antidepressants.
TABLE 1.
Prevalence of Use of Nonglycemic Medications Among Commercially Insured Adults Age ≥20 Years With Type 1 Diabetes, by Age, U.S., 2019
Women tended to use more nonglycemic medications overall—3.1 medications on average compared to 2.4 for men—and had higher prevalence of use of more medication categories (diuretics, beta blockers, central nervous system agents, psychotherapeutics, antidepressants, and hormones) than men (Table 2, Appendix Table A1). Exceptions to this sex imbalance included ACE inhibitors and statins, which were more often used by men.
TABLE 2.
Prevalence of Use of Nonglycemic Medications Among Commercially Insured Adults Age ≥20 Years With Type 1 Diabetes, by Sex, U.S., 2019
For the use of nonglycemic medications by race and ethnicity, Hispanic adults with type 1 diabetes were less likely to have prescriptions filled for statins compared to White and Asian adults with type 1 diabetes (29.7% vs. 35.9% and 34.9%, respectively) (Table 3, Appendix Table A1). White adults were more likely to have prescriptions filled for antidepressants compared to adults of all other race and ethnicity groups (21.5% vs. 17.5% for Black, 14.7% for Hispanic, and 12.6% for Asian adults). White and Black adults were more likely to have prescriptions filled for any antihypertensive medication compared to Hispanic and Asian adults (40.9% and 42.5% vs. 36.7% and 36.8%, respectively).
TABLE 3.
Prevalence of Use of Nonglycemic Medications Among Commercially Insured Adults With Type 1 Diabetes, by Race and Ethnicity, U.S., 2019
Use of Technology for Diabetes Treatment and Management
Diabetes technology has become a major component of type 1 diabetes therapy. The use of continuous glucose monitors (CGM) is becoming the more common mode of glucose monitoring for most adults with type 1 diabetes. In a survey of more than 48,000 American adults and children with type 1 diabetes, by 2022, 78% were using CGM (26); racial and ethnic disparities were identified with CGM use (27). Traditionally, randomized trials assessing glycemic benefits between insulin pumps and multiple daily injections of insulin have shown inconsistent results. However, with the concomitant use of insulin pumps and continuous glucose monitoring, hybrid closed loop system therapy has evolved rapidly, and its use is increasing in adults with type 1 diabetes.
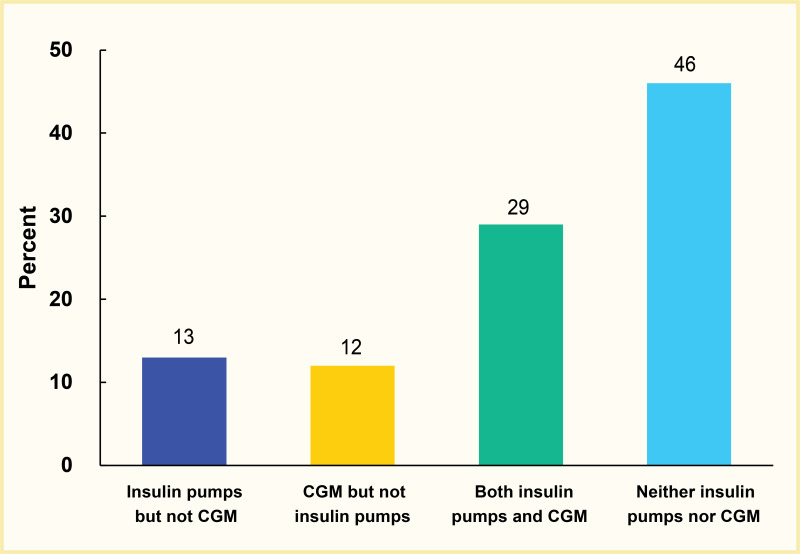
In a 2020 study using the IBM MarketScan commercial database, 42% of adults with type 1 diabetes were using insulin pump therapy, 41% were using CGM, and 29% were using both insulin pump and CGM in this commercially insured population (Figure 5) (3).
FIGURE 5.
Diabetes Technology Use Among Commercially Insured Adults Age ≥18 Years With Type 1 Diabetes, U.S., IBM MarketScan Database, 2020. CGM, continuous glucose monitor.
Type 1 Diabetes in Youth
Overview
Type 1 diabetes is one of the most common chronic diseases of youth (28), and the incidence of youth-onset type 1 diabetes has been increasing in the United States. Data from SEARCH, a population-based registry study conducted in five states (5), demonstrated that the incidence of type 1 diabetes in youth diagnosed at age <20 years increased from 19.5 per 100,000 in 2002–2003 to 22.2 per 100,000 in 2017–2018 (7,29). Increases in type 1 diabetes incidence were significantly greater in Asian or Pacific Islander, non-Hispanic Black, and Hispanic youth than in non-Hispanic White youth. The prevalence of type 1 diabetes in U.S. youth has also increased, from 1.48 per 1,000 youth in 2001 to 2.15 per 1,000 in 2017, based on SEARCH data (30). Having type 1 diabetes diagnosed in childhood predisposes complications in young adulthood, including kidney disease, retinopathy, peripheral neuropathy, and cardiovascular disease (31). Additionally, acute complications of diabetic ketoacidosis and severe hypoglycemia in youth with type 1 diabetes can result in morbidity and mortality (32,33).
Despite advances in technology, average A1C remains above the target of 7.0% (53 mmol/mol) in youth with type 1 diabetes, with a mean A1C of 8.4% (68 mmol/mol) at ages 1–9 years, 9.1% (76 mmol/mol) at ages 10–14 years, and 9.3% (78 mmol/mol) at ages 15–19 years after adjustment for demographic and clinical factors, including diabetes duration, in the 2014–2019 SEARCH cohort (Figure 6) (6). In the 10–14 and 15–19-year age groups, A1C levels increased from previous years’ cohorts (Figure 6) (6). However, a publication from the U.S. T1D Exchange reported a decrease in mean A1C among persons with type 1 diabetes receiving care in 3 adult and 12 pediatric centers across the United States when comparing data from 2016–2017 and 2021–2022 (26); this finding is encouraging, although the changes were nonsignificant. Mean A1C decreased from 8.8% to 8.6% (73 to 70 mmol/mol) and from 9.2% to 8.6% (77 to 70 mmol/mol) at ages 1–15 years and 16–25 years, respectively (26).
Technology use, including insulin pumps, CGM, and hybrid closed loop systems, continues to lag in non-Hispanic Black and Hispanic youth relative to non-Hispanic White youth (34,35).
Antihyperglycemic Medications
The mainstay of treatment for type 1 diabetes in youth remains insulin; however, the use of adjunctive antihyperglycemic medications that are primarily used for type 2 diabetes has been explored in persons with type 1 diabetes (36). In clinical trials in adults with type 1 diabetes, many of these adjunctive therapies improved glycemic control, decreased hypoglycemia, and lowered cardiovascular risk (36). Sparse data are available on the benefits of these medications in youth with type 1 diabetes.
Metformin is the most studied adjunctive therapy in youth with type 1 diabetes. In one meta-analysis, metformin decreased body mass index (BMI) and total daily insulin dose but was associated with a significantly increased risk of hypoglycemia (risk ratio 3.3, 95% CI 1.05–9.32, p=0.04) in adolescents with type 1 diabetes (37). There are few studies using adjunctive therapies, such as GLP-1 RAs, in youth with type 1 diabetes (38,39), and results are inconclusive to date. While adjunctive therapies may be used to treat obesity in youth with type 1 diabetes, their use as adjunctive therapy for glycemic management is considered off-label, and prevalence of their use is not well studied. In the T1D Exchange 2016–2018 (8), no use of adjunctive medications was reported in youth age <6 years, and use was <1% at age 6–12 years (metformin only). Among youth age 13–17 years with type 1 diabetes, 3% were on metformin, and <1% each were on a GLP-1 RA, DPP-4 inhibitor, SGLT2 inhibitor, or pramlintide. In SEARCH, among youth and young adults with type 1 diabetes, use of an oral agent in addition to insulin ranged from 2.6% to 3.3% over study time periods from 2002 to 2019 (6).
Medications for Other Health Conditions
Youth with type 1 diabetes may have comorbidities and complications of diabetes, and medications for these health conditions may be required (31). Comorbidities include mental health conditions, such as anxiety, depression, and disordered eating, as well as cardiovascular risk factors, such as hyperlipidemia and hypertension (40,41,42,43). Complications of diabetes potentially seen in childhood include nephropathy, neuropathy, and retinopathy (31). There are limited data on the prevalence of medication usage for these comorbidities and complications in U.S. youth with type 1 diabetes; data that are available are limited to single centers or studies and, thus, are not generalizable to the U.S. type 1 diabetes population. The current prevailing sentiment is that pediatric providers are less aggressive in treatment of dyslipidemia or hypertension compared to providers who care for adults with type 1 diabetes (44,45).
Use of Technology for Diabetes Treatment and Management
The mainstay of management of type 1 diabetes in youth remains glucose monitoring and subcutaneous insulin administration. However, the advent of new technologies, particularly automated insulin delivery (AID), has rapidly changed the landscape of diabetes management. CGM devices allow accurate and frequent monitoring of glucose levels, providing not only current estimation of glucose levels, but also trends, to help individuals make better real-time decisions around insulin administration, as well as decrease the burden of finger sticks (46,47). Insulin pumps administer insulin subcutaneously without the need for multiple daily injections and with the ability of more fine-tuned insulin delivery with differential basal rates, various bolus delivery methods, and other features (48). Further, these two technologies are integrated in AID systems, also known as hybrid closed loop systems, where CGMs communicate directly with insulin pumps and insulin administration is adjusted algorithmically based on glucose trends and predictions (48).
This technology for diabetes management has been associated with improvement of metabolic control. AID was associated with the lowest A1C in a single-center cohort of youth with type 1 diabetes in Denver, Colorado (49). In this study, CGM use was associated with lower A1C regardless of insulin delivery method; compared to multiple daily injections, insulin pump use was only associated with lower A1C when combined with CGM use (49). Other data demonstrate the benefits of AID therapy in youth, including improved A1C and diabetes-specific quality of life (50,51,52). For example, in a 6-month randomized clinical trial of AID therapy in Australia, time in range increased by 6.2% and diabetes-specific quality of life improved significantly (50). In addition, very young children benefit from AID therapy with improved time in range and no increase in hypoglycemia or diabetic ketoacidosis (51).
There has been rapid uptake of diabetes technologies in U.S. pediatric populations. In the SEARCH population study of youth and young adults with type 1 diabetes, the distribution of insulin regimens shifted from 24.9% insulin pump, 29.4% basal-bolus multiple daily injections, and 45.5% other (three or fewer insulin injections per day) in 2002–2007 to 49.1% insulin pump, 44.8% basal-bolus multiple daily injections, and only 4% other in the 2014–2019 cohort (6). An analysis of the T1D Exchange registry 2016–2018, using data from 81 U.S. pediatric and adult endocrinology practices, reported that across the pediatric age groups 1–5, 6–12, and 13–17 years, 64%, 68%, and 62%, respectively, of youth with type 1 diabetes used insulin pumps and 51%, 37%, and 24% used CGM (Table 4) (8). Both CGM and insulin pump use increased over time across all age groups, which represents a promising trend (Figure 7). CGM use was highest in the 1–5-year-old age group, and insulin pump use was highest in the 6–12-year-old age group.
TABLE 4.
Demographic Characteristics and Technology Use in Youth Age <18 Years With Type 1 Diabetes, by Age, T1D Exchange Registry, 2016–2018
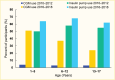
FIGURE 7.
Trends in Technology Use Among Youth Age ≤18 Years With Type 1 Diabetes, T1D Exchange Registry, 2010–2012 vs. 2016–2018. CGM, continuous glucose monitor.
In 2016, the first AID system became available for commercial use in the United States, and since then, two additional systems have been released. Use of AID is increasing quickly, with 33% of all participants in the T1D Exchange using this technology in 2021–2022; nonetheless, disparities exist by race, ethnicity, and type of health insurance (26). For example, a higher percentage of non-Hispanic White youth use CGMs and insulin pumps compared to non-Hispanic Black or Hispanic youth (8). Disparities also exist around household income, in that those with household income ≥$75,000 have higher technology use compared to those with lower household income; this difference is seen across race and ethnicity categories (8).
Management of type 1 diabetes requires frequent glucose monitoring, and frequency of glucose monitoring strongly correlates with A1C (53). The American Diabetes Association (ADA) recommends that youth with type 1 diabetes monitor their blood glucose frequently—up to six to ten times per day if not using CGM (54). This recommendation stems from data from the T1D Exchange clinic registry, where a higher frequency of blood glucose self-monitoring was associated with lower A1C levels (55). Figure 8 depicts data on glucose self-monitoring frequency in youth from the T1D Exchange registry who were not using CGM. In 2016–2018, only about one-half of youth age 1–12 years monitored their blood glucose four to six times per day, with that number dropping to less than one-half after age 12 years (8).
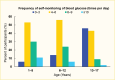
FIGURE 8.
Self-Monitoring of Blood Glucose Among Youth Age 1–17 Years With Type 1 Diabetes, T1D Exchange Registry, 2016–2018. Data are derived from youth who were not using a continuous glucose monitor.
Self-Care Practices
Self-care practices for type 1 diabetes include attention to diet and exercise, in addition to glucose monitoring and insulin administration. Adherence to dietary and exercise recommendations for youth and young adults with type 1 diabetes tends to be poor, even more so for youth in households with food insecurity (56).
Depending on the age at diagnosis, youth with type 1 diabetes often have variable degrees of independence and autonomy regarding diabetes self-management, and self-management increases as youth mature (57). Parents often play a large role in diabetes management, particularly at young ages. In some circumstances, such as developmental delays, youth may not achieve full independence in diabetes self-care. Youth and their families are each unique in their transition to self-care. Diabetes burnout in youth may lead to parents or other caregivers stepping back in to assist with care (58).
An organized and planned transition with education and psychosocial support starting from early adolescence is crucial to help youth with type 1 diabetes achieve a successful transition to adult medical care. For example, the Helmsley T1D Transition Let’s Empower and Prepare (LEAP) Program was a structured transition program for youth with type 1 diabetes led by a multidisciplinary team consisting of a certified diabetes educator, registered dietitian, and medical provider (59). Unique benefits of the LEAP Program included development-tailored diabetes education and the use of case managers who coordinated transfer from the pediatrics clinic to the adult clinic and encouraged adherence to scheduled visits. A prospective, nonrandomized trial that compared the efficacy of the LEAP Program to standard care showed that participants who participated in the structured transition program had improved glycemic control, incidence of severe hypoglycemia, and psychosocial well-being at 12 months.
Type 2 Diabetes in Adults
Overview
While the incidence of diabetes has shown a gradual decline over the past two decades, the prevalence of total diabetes (diagnosed and undiagnosed) in adults increased from 10% in 2001–2002 to 13.1% in 2017–2020, with the majority having type 2 diabetes (60). This increase in prevalence has been observed across all age, sex, and racial and ethnic subgroups, with persistent disparities as racial and ethnic minority groups have a higher prevalence of diabetes compared to non-Hispanic White persons. Individuals with diabetes often experience multiple comorbidities. In addition to maintaining proper glycemic control, managing these comorbidities is crucial to prevent severe complications and improve overall health outcomes.
Apart from making lifestyle changes, the use of medications, including non-insulin antihyperglycemic medications, insulin, and medications to treat diabetes-related comorbidities, has become essential in diabetes management. The advent of newer diabetes drugs has brought about significant advancements in treatment. These medications not only help in reducing blood glucose levels but also offer additional benefits, such as improved renal function, cardiovascular protection, and even a reduction in mortality rates (61). This marks a positive shift in diabetes management, where the focus extends beyond glucose control to encompass broader health benefits. By understanding the advancements in diabetes medications and the importance of managing comorbidities, healthcare professionals can provide more comprehensive care to individuals with diabetes, ultimately leading to better health outcomes.
Antihyperglycemic Medications
NHANES data collected between 2015 and March 2020 show that 81.8% of participants age ≥20 years with type 2 diabetes took an antihyperglycemic medication: 57.7% were taking non-insulin medications alone, 6.3% were taking insulin alone, and 17.8% were taking a combination of insulin and non-insulin antihyperglycemic medications (Figure 9). Adults age <45 years, women, Hispanic individuals, those having less than a high school education level, or those with a poverty income ratio of <1.00 were more likely to report not taking any antihyperglycemic medications (Table 5) compared to their demographic counterparts. Antihyperglycemic medication use increased with increasing A1C level—that is, the higher the A1C, the more likely the individuals used insulin alone or combination therapy. Similar patterns were seen among individuals with a diabetes duration of ≥10 years compared to <10 years. Regarding the number of medications, one-half of adults with type 2 diabetes received one medication and the remaining half received two or more antihyperglycemic medications (Table 6). More non-Hispanic White persons used three or more medications compared to non-Hispanic Black persons.
TABLE 5.
Prevalence of Use of Antihyperglycemic Medications Among Adults Age ≥20 Years With Diagnosed Type 2 Diabetes, U.S., 2015–March 2020
TABLE 6.
Distribution of Number of Antihyperglycemic Medications Among Adults Age ≥20 Years With Diagnosed Type 2 Diabetes Who Take Any Diabetes Medications, U.S., 2015–March 2020
Among NHANES participants with type 2 diabetes who were treated with antihyperglycemic medication in 2015–March 2020, the most common drug class was biguanide (i.e., metformin) (63%), followed by sulfonylurea (25.6%) and insulin (22.3%), whereas thiazolidinedione was the least used medication (4.3%) (Table 7). Fewer than one-half (41.9%) of adults with diagnosed type 2 diabetes were on monotherapy with one of the seven major drug classes, among whom 70% received metformin. Metformin, sulfonylureas, and insulin were also the top three medications for patients on combination therapy. Use of antihyperglycemic drug classes differed by participant characteristics. Older age was associated with higher frequency of sulfonylurea and insulin use. Use of thiazolidinedione and DPP-4 inhibitors was also higher in those age ≥45 years compared to younger individuals. In contrast, use of GLP-1 RAs and SGLT2 inhibitors was more common in individuals age 20–44 and 45–64 years (range 5%–8%) compared to those age ≥65 years (range 2%–4%). Insulin use did not differ by sex, but men were more often treated with medications for all other drug classes than women. Compared to non-Hispanic White individuals, non-Hispanic Black individuals had a higher prevalence of using insulin but lower prevalence of all other medications, while Hispanic persons were less likely to use any medication.
TABLE 7.
Prevalence of Use of Antihyperglycemic Medications Among Adults Age ≥20 Years With Diagnosed Type 2 Diabetes, U.S., 2015–March 2020
Trends in Antihyperglycemic Medication Use
Among NHANES participants, the age-standardized prevalence of adults age ≥20 years with type 2 diabetes receiving no medication remained stable between 1999–2004 and 2017–March 2020 (Figure 10). The age-standardized prevalences of using insulin alone and non-insulin antihyperglycemic medications alone decreased, while that of using combination therapy—insulin plus non-insulin antihyperglycemic medications—increased. The numbers of people using no medication, non-insulin medications only, or both insulin and non-insulin medications increased from 1999–2004 to 2017–March 2020 (Figure 11). The number of people using insulin alone remained stable. A published study showed that when stratifying by the number of medications used, the prevalence of participants using one medication increased, while that of people using two or more medications remained unchanged from 2003–2004 to 2015–2016 (62). Of note, the NHANES did not include information about lifestyle modification (diet and physical activity) for treating diabetes. Therefore, it was unclear whether participants who did not report being treated with antihyperglycemic medications were deliberately being managed exclusively with diet and lifestyle modification. Among those who reported not taking any antihyperglycemic medications in the NHANES 2017–2020, 78.8% had A1C <7.0%, 11% had A1C 7%–<8%, and 10.2% had A1C ≥8.0% (data not shown).
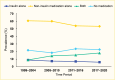
FIGURE 10.
Age-Standardized Prevalence of Adults Age ≥20 Years With Diagnosed Type 2 Diabetes, by Antihyperglycemic Medication Use, U.S., 1999–March 2020. Diagnosed type 2 diabetes is self-reported; probable type 1 diabetes (insulin use only and (more...)
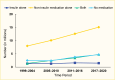
FIGURE 11.
Number of Adults Age ≥20 Years With Diagnosed Type 2 Diabetes, by Antihyperglycemic Medication Status and Time Period, U.S., 1999–March 2020. Diagnosed type 2 diabetes is self-reported; probable type 1 diabetes (insulin use only and continuous (more...)
Trends in medication use by drug classes reflect the change in clinical practice over time due to evidence of their relative benefits and risks, introduction of new pharmacologic options (e.g., GLP-1 RAs and SGLT2 inhibitors), cost, change in healthcare coverage, medical care guidelines, and patient and provider preferences. Among NHANES participants age ≥20 years with diagnosed diabetes, use of metformin, insulin, DPP-4 inhibitors, SGLT2 inhibitors, and GLP-1 RAs increased significantly from 1999–2002 to 2015–2018 (63). Meanwhile, the prevalence of sulfonylurea use decreased substantially, as did that of thiazolidinediones. When stratifying by monotherapy versus combination therapy, only metformin monotherapy increased among participants on monotherapy, doubling in 2015–2016 compared to 2003–2004 (62). In participants on combination therapy (two or more medications), use of metformin remained stable, while use of insulin, DPP-4 inhibitors, SGLT2 inhibitors, and GLP-1 RAs all increased.
Among one million patients newly diagnosed with type 2 diabetes who were identified from the 2005–2016 Centricity Electronic Medical Records, including U.S. primary and secondary ambulatory care systems, metformin was the most commonly initiated therapy (11). The share of metformin increased significantly from 2005 to 2016, and its rate of discontinuation within one year was low (8%). Despite a decrease in use over time, sulfonylureas were still the most popular second-line agent. Similar findings were reported in a study using Medicare and commercial insurance databases (64). In 2019, although the use of SGLT2 inhibitors and GLP-1 RAs was generally low, their use increased among individuals with cardiovascular disease due to recent evidence of their cardiovascular benefits (64).
Among people new to insulin, use of long-acting insulin increased almost twofold from 2007 to 2014 in Medicare patients (65). While use of short-acting and combination insulin regimens decreased over time, 16% and 27% of individuals, respectively, were using these regimens in 2014. Individuals age >75 years or having three or more comorbidities had higher prevalences of using short-acting and combination regimens compared to younger adult patients and those with fewer comorbidities. Among adult ambulatory care diabetes patients of all ages, long-acting insulin was prescribed in two-thirds of all outpatient visits in 2020 (66); the most used insulin was insulin glargine, representing half of all insulin used in 2016–2020.
The introduction of new antihyperglycemic medications that result in lower A1C, as well as benefits for heart failure and cardiovascular and renal disease, has changed the paradigm in type 2 diabetes care. However, given that newer diabetes drugs are often more expensive and have inconsistent health insurance coverage, treatment disparities exist. Using Medical Expenditure Panel Survey 2003–2019 data, the prevalence of using DPP-4 inhibitors, SGLT2 inhibitors, and GLP-1 RAs was examined across demographic and socioeconomic subgroups (67). Over the study period, disparities in use increased, with increasing difference in prevalence of use of these three drug classes in Black compared to White adults. Specifically, 0.9% of Black and 2.2% of White adults used these medications in 2003–2006 compared to 18.3% of Black and 22.6% of White adults in 2017–2019. By health insurance status, the difference in prevalence of use in uninsured individuals compared to those with private health insurance also increased over time (1.8% and 2.1% in 2003–2006 vs. 17.6% and 25.4% in 2017–2019, respectively). Systematic approaches may be needed to address the gaps in access to newer diabetes drug classes.
Medications for Other Health Conditions
Individuals with type 2 diabetes have a high prevalence of diabetes-related comorbidities, including various macrovascular and microvascular complications (68). Most individuals with type 2 diabetes have at least one more comorbidity, while almost one-half have two or more coexisting conditions (69). Common comorbidities include hypertension, hypercholesterolemia, and chronic kidney disease. Failure to control diabetes-related comorbidities can lead to the development of more severe complications, whereas optimizing management of glucose, blood pressure, lipids, and smoking will help reduce risks of atherosclerotic cardiovascular disease (ASCVD), blindness, kidney failure, and amputations.
Individuals with diabetes often use multiple concomitant medications. In the NHANES 2015–March 2020, participants age ≥20 years with diagnosed type 2 diabetes used 5.2 prescription drugs on average compared to 3.2 and 3.1 prescription drugs in people with undiagnosed diabetes (A1C ≥6.5% [≥48 mmol/mol] or FPG ≥126 mg/dL [≥7.00 mmol/L]) and those with prediabetes (A1C 5.7%–6.4% [39–46 mmol/mol] or FPG 100–125 mg/dL [5.55–6.94 mmol/L]), respectively (Figure 12, Table 8, Appendix Table A1). In addition, medication use was almost twice as high in participants with diagnosed type 2 diabetes compared to those with normal glucose levels (A1C <5.7% and FPG <100 mg/dL).
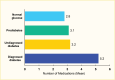
FIGURE 12.
Age-Standardized Mean Number of Medications Among Adults Age ≥20 Years, by Diabetes Status, U.S., 2015–March 2020. Medication classes (see Appendix Table A1) include diuretics, angiotensin II receptor blockers (ARBs), calcium channel blockers (more...)
TABLE 8.
Age-Standardized Prevalence of Diabetes Comorbidities and Associated Medication Use Among Adults Age ≥20 Years, by Diabetes Status, U.S., 2015–March 2020
Hypertension Treatment
Hypertension is the most common comorbidity in individuals with type 2 diabetes, with an estimated prevalence of up to 73.6% (70). Among NHANES 2015–March 2020 adult participants age ≥20 years, the age-standardized prevalence of self-reported hypertension was highest among adults with diagnosed type 2 diabetes (59.4%) compared to those with undiagnosed diabetes (46.8%), prediabetes (39.4%), or normal glucose levels (27.1%) (Table 8). In patients receiving care in a large health system in 2013, 87% of patients with type 2 diabetes had hypertension documented in their electronic health record (71).
Maintaining blood pressure <140/90 mmHg decreases the risk of ASCVD, heart failure, and microvascular complications in individuals with type 2 diabetes (72,73,74,75). In addition to lifestyle intervention, medications can be used to achieve the optimal blood pressure target. Drug classes that are associated with a reduction of ASCVD events include ACE inhibitors, ARBs, thiazide-like diuretics, and dihydropyridine calcium channel blockers (76,77,78,79).
Consistent with the prevalence data, use of antihypertensive medication increased with increasing A1C. In the NHANES 2015–March 2020, almost 60% of adults age ≥20 years with diagnosed type 2 diabetes took one or more antihypertensive medication, which was 1.5, 2, and 3 times higher than participants with undiagnosed diabetes, prediabetes, and normal glucose levels, respectively (Table 8, Appendix Table A1). The most common antihypertensive drug class used in adults with diagnosed diabetes was ACE inhibitors, followed by diuretics, beta blockers, and then ARBs. In contrast, diuretics and beta blockers were the most commonly used drugs in adults with undiagnosed diabetes, prediabetes, and normal glucose levels.
Cholesterol Treatment
Hypercholesterolemia is another common comorbidity in individuals with type 2 diabetes. Among NHANES 2015–March 2020 participants, half (52.8%) of adults age ≥20 years with diagnosed type 2 diabetes reported having hypercholesterolemia, while the age-standardized prevalence was lower in the undiagnosed diabetes (37.8%), prediabetes (36.2%), and normal glucose (29.6%) groups (Table 8).
Like hypertension, hypercholesterolemia is an important risk factor for ASCVD. A strong management plan will help decrease the risk of adverse health outcomes. Lifestyle interventions, such as weight loss, physical activity, a Mediterranean style or Dietary Approaches to Stop Hypertension (DASH) diet, are effective at reducing ASCVD risk in some individuals (80,81). In addition, statins reduce the incidence of ASCVD and all-cause and vascular mortality in individuals with diabetes (82,83,84,85,86). Therefore, use of high-intensity statin therapy for secondary prevention of ASCVD in diabetes patients at high risk and moderate-intensity statin therapy for patients with diabetes age ≥40 years without known ASCVD are generally recommended (23,87). Combination of statins and other lipid-lowering medications, such as ezetimibe and PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors, is often reserved for individuals with diabetes who do not tolerate statins and those with established ASCVD.
In the NHANES 2015–March 2020, statins were the most commonly used cholesterol medication among adults with type 2 diabetes (43.0%). Coupled with a lower prevalence of reported hypercholesterolemia, use of cholesterol medication was also lower in adults with undiagnosed diabetes, prediabetes, and normal glucose levels than in those with diagnosed type 2 diabetes (Table 8, Appendix Table A1).
Other Conditions
Given that individuals with type 2 diabetes have many coexisting morbidities, it is not surprising that they take multiple medications in addition to those for controlling glycemia, blood pressure, and lipids. In the NHANES 2015–March 2020, almost one-third of individuals with diagnosed type 2 diabetes used medications for the central nervous system (e.g., analgesics, anticonvulsants, antiemetic/antivertigo, or antiparkinsonian agents), and one in five used psychotherapeutic agents, antidepressants, or hormones (Table 8, Appendix Table A1). Again, the age-adjusted prevalence of using other prescribed medications was higher in individuals with diagnosed type 2 diabetes than in those with undiagnosed diabetes, prediabetes, and normal glucose levels.
Aspirin Use
For diabetes patients with a history of ASCVD, use of aspirin for secondary prevention is generally recommended (88). Aspirin is not currently recommended for primary prevention due to the risk of bleeding, which outweighs the ASCVD benefit (88,89,90,91). Therefore, use of aspirin for primary prevention is currently recommended for patients age ≥50 years with one or more major ASCVD risk factors and low bleeding risk (87). For individuals age >70 years, aspirin is generally not recommended because the increased risk of bleeding outweighs the benefit (92).
In the NHIS 2019, more than one-half (57.8%) of U.S. adults age ≥40 years who reported having type 2 diabetes were medically advised to take low-dose aspirin, of whom fewer than half were currently taking low-dose aspirin (47.4%) (Table 9). In contrast, almost one in ten patients with type 2 diabetes who were not advised to take aspirin were currently taking low-dose aspirin on their own. Individuals age ≥65 years, men, non-Asians, and those with diabetes duration ≥10 years or with a history of ASCVD were more likely to be advised to take low-dose aspirin than younger patients, women, Asians, or those with diabetes duration <10 years or without a history of cardiovascular disease, respectively. Among patients who took aspirin on their own, it is noteworthy that non-Hispanic Black adults and those with diabetes duration <10 years or without a cardiovascular disease history tended to use aspirin more often than other groups (Table 9).
TABLE 9.
Prevalence of Low-Dose Aspirin Use Among Adults Age ≥40 Years With Diagnosed Type 2 Diabetes, U.S., 2019
Glucose Monitoring and Associated Technology
Glucose monitoring is an integral part of an effective diabetes management plan for individuals on insulin therapy. With glucose self-monitoring, individuals can adjust their diet, activity, and medications to meet glucose targets and recognize and treat hypoglycemia and hyperglycemia. Hence, glucose monitoring could help prevent both hypo- and hyperglycemia. The frequency and timing of glucose monitoring should be individualized and evaluated regularly by health care providers.
In individuals on intensive insulin therapy, glucose monitoring is important for maximizing treatment benefits while reducing risk associated with hypoglycemia. While glucose monitoring is critical in patients with type 1 diabetes, its utility in those with type 2 diabetes using basal insulin with or without other non-insulin agents is less evident. In a randomized controlled trial (RCT), glucose monitoring improved glycemic control and decreased risk of hypoglycemia in participants with type 2 diabetes requiring basal insulin (93). However, in participants with well-controlled type 2 diabetes who were not on insulin, glucose monitoring did not have additional benefit (94,95). To realize its full benefit in individuals with type 2 diabetes, glucose monitoring must be used in conjunction with a management plan. When used in a structured fashion to adjust treatment therapy, glucose monitoring led to a greater reduction of glucose readings versus when not using glucose monitoring (96).
Compared to self-monitoring of blood glucose with a glucose meter, the use of CGMs, which transmit data to a receiver that provides real-time information and actionable alerts, over a duration of 8 and 24 weeks resulted in lower A1C levels in individuals with type 2 diabetes who had a high A1C and were treated with basal insulin without prandial insulin (97,98,99). In those receiving multiple daily insulin injections, CGM for 24 weeks was also better than usual care in controlling glycemia (100). Intermittently scanned CGM, which requires users to scan a sensor to get data from the system, is another option for glucose monitoring in patients with type 2 diabetes, but RCTs examining its effectiveness have been limited and inconsistent (101,102,103). In contrast, observational studies consistently showed the benefit of intermittently scanned CGM on glycemic control in patients with type 2 diabetes (104,105). The choice of glucose monitoring method and device should depend on an individual’s circumstances. Regardless of devices selected, continued education and training are keys to optimal use. Medicare now covers CGMs for patients who need to check their blood glucose levels four or more times per day and either use an insulin pump or have three or more insulin injections daily.
Data on glucose monitoring adherence in individuals with type 2 diabetes are limited. A 2023 study on Medicare beneficiaries found that more than 70% of individuals with type 2 diabetes who were on insulin either did not monitor their glucose or had <80% of days covered with a prescription of glucose monitoring supplies (meeting the Centers for Medicare and Medicaid Services definition of nonadherence) (106).
Self-Care Practices
Individuals with diabetes can reduce the risk of developing serious and costly diabetic complications by engaging in evidence-based self-care and clinical preventive practices. Appropriate glucose monitoring, regular tests for A1C, diabetes self-management education and support (DSMES) classes, medical nutrition therapy, physical activity, smoking cessation, foot and eye exams, and routine vaccinations are among good self-care management activities that can help individuals with diabetes attain target A1C, detect diabetes-related complications early, decrease the risk of infections, improve quality of life, and reduce associated treatment costs (107,108).
Data from the Nurses’ Health Study and Health Professionals Follow-up Study suggested that adherence to healthy lifestyle behaviors was suboptimal in patients with type 2 diabetes (109). In the study, low-risk lifestyle factors were defined as (1.) a diet score in the upper 40th percentile of the study population, (2.) BMI between 18.5 and 25 kg/m2, (3.) noncurrent smoking, (4.) alcohol use of 5–15 grams per day for women and 5–30 grams per day for men, and (5.) >150 minutes per week of moderate-to-vigorous activities. The authors found that fewer than one in four patients with type 2 diabetes had three or more low-risk lifestyle factors at diagnosis.
In 2015–March 2020, 88% of NHANES participants with type 2 diabetes reported having their A1C checked, while 73% had a foot exam and 65.5% had an eye exam in the past year (Table 10). Almost two-thirds and more than one-half of participants performed a self-exam of their feet and self-monitoring of blood glucose daily, respectively. In addition, one in four participants saw a certified diabetes educator (i.e., diabetes nurse educator or dietitian or nutritionist), and 8 in 10 saw a health care professional for diabetes (doctor or other health care provider other than diabetes educators, dietitians, or foot and eye doctors) in the past year. Individuals age ≥65 years were more likely to receive foot and eye exams and A1C tests and to see a health care professional for diabetes, but not a certified diabetes educator, compared to younger patients, while use of the remaining services did not differ by age. In addition, a higher proportion of non-Hispanic White adults, those with education above high school, and those with health insurance had their A1C checked in the last year or had seen a health care professional for diabetes compared to persons of other races and ethnicities, those with less than a high school education, and those without health insurance, respectively. Individuals who have had diabetes ≥10 years were more likely to perform self-care practices than those with <10 years of diabetes duration.
TABLE 10.
Prevalence of Self-Reported Preventive Care Practices Among Adults Age ≥20 Years With Diagnosed Type 2 Diabetes, U.S., 2015–March 2020
A study among adult participants of the Behavioral Risk Factor Surveillance System 2017–2018 who reported having diabetes showed that 9 out of 10 saw a health professional for diabetes in the past year (110). About one-half of participants attended a course or class in diabetes management, and attendance was associated with higher engagement in self-care practices compared to those who did not attend a class.
Trends in Self-Care Practices
Using the Behavioral Risk Factor Surveillance System 2011–2018 with self-reported data, the proportion of adults age ≥18 years with diagnosed diabetes receiving two or more A1C tests, a foot exam by health care professional, or a dilated eye exam in the last year has increased slightly (Figure 13) (60,110,111). Specifically, the percentage of patients with two or more A1C tests annually increased from 68.8% in 2011 to 75.2% in 2017–2018, which was similar to the increase in the percentage of patients with a foot exam. A little more than two-thirds of patients received a dilated eye exam in 2017–2018, an increase of 9 percentage points compared to 2011. Of note, the proportion of patients ever attending a diabetes management class and who self-monitored their blood glucose daily decreased almost 4 percentage points over time. Based on data from the U.S. Diabetes Surveillance System, the rate of influenza or pneumococcal vaccination among adult patients with diabetes did not improve; a little more than one-half and one-third of patients received these vaccines each year, respectively (111).
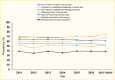
FIGURE 13.
Prevalence of A1C Testing, Diabetic Complications Screening, Home Glucose Testing, and Vaccinations Among Adults Age ≥18 Years With Diagnosed Diabetes, U.S., 2011–2018. Diagnosed diabetes is self-reported. Data are age-standardized to (more...)
The quality of diet among U.S. adults with diabetes decreased from 1999 to 2016 (112). The proportion of individuals with <10% total daily calories from saturated fats, <2,300 mg sodium per day, ≥14 g fiber per 1,000 kcal, or <300 mg cholesterol per day decreased over time. On the other hand, engagement in aerobic physical activity increased. On average, U.S. adults with diabetes exercised 103.3 minutes per week in 2011–2016 compared to 86.4 minutes in 1999–2004. Therefore, fewer individuals had <10 minutes and more individuals had ≥150 minutes of aerobic activity per week in 2016 than 1999 (112).
Barriers to Self-Care
Individuals with diabetes face many challenges in managing their diabetes and practicing self-care in daily life. DSMES increases patient adherence to best practice recommendations (113); however, <10% of Medicare or privately insured patients received DSMES due to various health system, logistic, and programmatic barriers (114,115). The need perceived by providers and patients may also play a role. Although good nutrition is an indispensable component of a diabetes management plan, individuals with diabetes often find it difficult to choose appropriate foods (116). For some individuals with type 2 diabetes who need medical nutrition therapy, a lack of referrals to dietitians or DSMES contributes to the low participation (108,117). Addressing access to DSMES, access to affordable, healthy, and high-quality food, barriers to behavioral changes, and health literacy may improve nutrition. Other societal interventions for the general population as recommended by the National Clinical Care Commission might also be helpful (118).
Individuals with diabetes could have diabetes distress and other psychological comorbidities that might affect self-management and treatment outcome (119). Psychosocial barriers can arise from inadequate family, social, and financial support, inaccurate self-perception of the benefits of treatment, inadequate problem-solving or coping skills, diabetes stigma, or competing needs for other medical conditions (120,121,122,123). In individuals with mental health issues, the quality of and support for diabetes care, emotional well-being, and beliefs of self-management ability and consequences of suboptimal diabetes control all contribute to lower engagement in diabetes self-management (124). Using different measures to assess an individual’s psychological health could help providers to identify important barriers to self-care and address them in a timely manner (119). A combination of approaches might be necessary to help decrease individuals’ distress and improve their psychological wellbeing, leading to better diabetes self-management and health outcomes (125,126).
Medication Adherence
If lifestyle changes fail to achieve the target A1C, individuals with type 2 diabetes will usually need to take medication for the rest of their lives (127). In these cases, medication adherence is important to achieve improved glycemic control, lower weight, lower risk of hypoglycemia, and higher quality of life (128,129,130,131). While nonadherence could lead to higher residual risks of macrovascular and microvascular complications even after intensive glycemic control and increased all-cause mortality, medication adherence results in lower health care utilization and saves costs (132,133,134,135,136,137). For example, adherence to insulin pen therapy is associated with decreased medical costs in privately insured and Medicaid patients with type 2 diabetes (138,139).
Medication nonadherence is common in patients with type 2 diabetes. Defining medication adherence as administering ≥80% of the amount of medications prescribed within an observational period (140), the prevalence of medication nonadherence was up to 32% for all oral medications in a meta-analysis and 53% for DPP-4 inhibitors among commercially insured patients (141,142). Nonadherence to insulin therapy is also prevalent, ranging from 20% of patients reporting regular insulin injection omission among internet-surveyed patients to 65% of commercially insured patients with <80% of days covered (143,144).
Several factors affect medication adherence. Individuals new to pharmacotherapy are less likely to adhere than experienced users (145). Younger age, lower educational level, and lower income are also associated with medication nonadherence. In addition, the high cost of insulin therapy and newer medication classes is a significant barrier to medication adherence (146,147,148,149). Low health literacy, food insecurity, and other social determinants of health are often not addressed during office visits despite being strongly associated with medication nonadherence (150,151,152,153). In elderly patients who use insulin, the type of device could affect adherence, with adherence improved by use of pen devices compared to insulin vials and syringes (154). Of all factors leading to medication nonadherence, potentially modifiable factors include an individual’s self-perception and beliefs of treatment efficacy, risks and benefits, experience with hypoglycemia, the ease of following a treatment regimen, out-of-pocket costs, and trust in physicians (155). Addressing these factors successfully is key to the development of effective clinical partnerships for optimal medication adherence.
Type 2 Diabetes in Youth
Overview
Type 2 diabetes has become increasingly common in youth in the United States, with its prevalence increasing from 0.34 to 0.67 per 1,000 youths between 2001 and 2017 (30). The relative annual increase in the incidence of type 2 diabetes in this time period was 4.8%, after adjusting for age, sex, race, and ethnicity (156). Additionally, youth from racial and ethnic minority groups are disproportionately impacted by type 2 diabetes, with the greatest rate of increase in Hispanic, Black, and American Indian/Alaska Native youth (156).
Type 2 diabetes is distinguished from autoimmune type 1 diabetes by the absence of islet autoantibodies. In addition, the development of type 2 diabetes almost always occurs after the onset of puberty, whereas the development of type 1 diabetes may occur before or after pubertal onset. Type 2 diabetes is also more commonly associated with overweight/obesity compared to type 1 diabetes; however, given the rising rates of obesity in the general population, one should not exclude a diagnosis of type 1 diabetes based on a child’s elevated BMI.
Importantly, type 2 diabetes in youth and adolescents has been reported to be a more aggressive disease and less responsive to metformin compared with type 2 diabetes diagnosed in adults. Compared to adults, youth and adolescents with type 2 diabetes have hyperresponsive beta cells, a faster decline in beta cell function (20%–35% per year), and lower insulin sensitivity. As a result, youth and adolescents with type 2 diabetes experience accelerated development of serious complications as young adults as they enter their most productive period (157,158).
The prevalence of comorbidities and complications in young adults who were diagnosed with type 2 diabetes during childhood is high, including high age-adjusted prevalence of diabetic kidney disease (19.9%), retinopathy (9.1%), peripheral neuropathy (17.7%), arterial stiffness (47.4%), and hypertension (21.6%) (31). In SEARCH, nearly three in four participants with type 2 diabetes had at least one diabetes-related comorbidity or complication (31). Similarly, the TODAY study reported at least one diabetes-related comorbidity in 60.1% of participants, and 28.4% of participants had two or more diabetes-related comorbidities; mean diabetes duration was 13.3±1.8 years (158). In SEARCH and the TODAY study, complications were more common among participants from minority race and ethnicity groups (31,158). Thus, type 2 diabetes is more aggressive in those with youth-onset versus adult-onset disease.
Antihyperglycemic Medications
Non-insulin Medications
Management of type 2 diabetes in youth includes both lifestyle modifications around diet and exercise, as well as medical therapy to promote weight loss and increase insulin sensitivity. Non-insulin medications that are currently approved by the U.S. Food and Drug Administration (FDA) for use in youth age <18 years with type 2 diabetes include metformin, the GLP-1 RAs, liraglutide, exenatide, and dulaglutide, and the SGLT2 inhibitor, empagliflozin (159,160). In the United States, many pediatric patients with type 2 diabetes do not meet glycemic targets. SEARCH did not find improvement of mean A1C in the 2014–2019 type 2 diabetes cohort compared to earlier cohorts (Figure 14) (6). This finding may partly be due to the more aggressive nature of youth-onset type 2 diabetes compared to adult-onset type 2 diabetes (54,159).
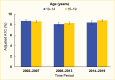
FIGURE 14.
Glycated Hemoglobin (A1C) Trends Among Youth Age 10–19 Years With Type 2 Diabetes, by Age and Time Period, SEARCH for Diabetes in Youth Study, 2002–2019. A1C values were adjusted for diabetes duration and are primarily derived from prevalent (more...)
Metformin, a biguanide drug, is the oldest and most prescribed antihyperglycemic agent that is FDA approved for children age ≥10 years with type 2 diabetes; metformin is recommended by the ADA as first line treatment of type 2 diabetes in asymptomatic children with A1C <8.5% (<69 mmol/mol) (54,161). Its mechanisms of action include reduction of hepatic gluconeogenesis, decrease of intestinal glucose absorption, and increase of insulin sensitivity, leading to decreased basal and postprandial glucose levels (161). Common side effects, such as nausea, vomiting, diarrhea, and dyspepsia, often limit its use in youth and may lead to the addition of other antihyperglycemic agents, such as GLP-1 RAs, SGLT2 inhibitors, and/or insulin, if target A1C is not attained on metformin alone.
Liraglutide, exenatide, dulaglutide, and empagliflozin are four additional drugs that are FDA approved for treatment of pediatric patients age ≥10 years with type 2 diabetes (160,162,163). Liraglutide, exenatide, and dulaglutide are GLP-1 RAs that stimulate glucose-dependent insulin release from the pancreatic islets, inhibit post-meal glucagon release, slow gastric emptying, and reduce appetite (164). Liraglutide is a daily injectable, whereas exenatide LA and dulaglutide are once-weekly injections. In randomized clinical trials that led to FDA approval of these drugs, GLP-1 RAs were used as an adjunct to a diet and exercise regimen to improve glycemic control (162,163,165). Their common side effects include gastrointestinal effects (nausea, vomiting, abdominal pain, and constipation) which may be dose-limiting, injection site reactions, and headache (162,163). More serious adverse effects include possible pancreatitis, hypoglycemia when used with insulin, acute gallbladder disease, and thrombocytopenia (only with exenatide). They also have a black box warning about the increased risk of thyroid C-cell tumors based on animal studies. Thus, GLP-1 RAs are not recommended for use in patients with a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia 2A or 2B. Empagliflozin is a SGLT2 inhibitor that promotes renal excretion of glucose by blocking proximal tubular glucose absorption and is administered orally once daily. Its adverse reactions include acute kidney injury, volume depletion, increased risk of genitourinary fungal infection, and euglycemic diabetic ketoacidosis.
Other antihyperglycemic agents that are approved for use in adults, such as sulfonylureas, alpha-glucosidase inhibitors, thiazolidinediones, and DPP-4 inhibitors (166), have been used off-label in pediatrics. Use of the medications was reported in approximately 15.3% of youth with type 2 diabetes in the SEARCH 2002–2007 cohort (6).
The TODAY study showed that metformin monotherapy provided durable glycemic control (A1C ≤8% [≤64 mmol/mol] for 6 months) in only about one-half of the participants enrolled (12). In the TODAY study, rosiglitazone and metformin in combination were more effective than either medication alone or lifestyle intervention alone. Thus, treatment of youth with type 2 diabetes may require concomitant use of multiple drugs, such as metformin, a GLP-1 RA, and insulin (162,163). The Ellipse study demonstrated that (single agent) liraglutide, in a dose up to 1.8 mg daily, is effective in managing pediatric type 2 diabetes, with a significant decrease in A1C of 0.64% compared to an increase of 0.42% in the control group (p<0.001) in a 26-week study, with no significant lowering of BMI z-score (162). Dulaglutide similarly was superior to placebo, with 51% of treated participants versus 14% in the control group achieving A1C <7.0% at 26 weeks; no between-group differences in the change in BMI were observed (165,167). Population-based studies of GLP-1 RA in the United States are not yet available. In 2022, the International Society of Pediatric and Adolescent Diabetes published new management guidelines for type 2 diabetes in youth, which include consideration of newer therapies (168).
Insulin
Basal insulin (starting at 0.5 units/kg/day) in combination with metformin is recommended by the ADA as part of treatment of youth with type 2 diabetes with A1C ≥8.5% (54). If A1C goals are not met with metformin and escalating doses of basal insulin, then prandial insulin may need to be added (54).
In the TODAY2 study, a 10-year observational follow-up study of the TODAY cohort, at a mean age of 21.7 years and diabetes duration of 8.1 years, 51% of participants were taking daily basal insulin (158). Data from SEARCH demonstrated that 23% of youth with type 2 diabetes with a mean diabetes duration of 7.0 years in 2014–2019 were on insulin only, around 24% were managed with insulin plus an oral agent, 24% were managed with metformin alone, 5% were on another oral agent, and about 22% were not on pharmacologic treatment (Figure 15) (6).
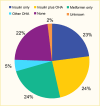
FIGURE 15.
Frequency of Antihyperglycemic Medications Used to Treat Youth-Onset Type 2 Diabetes at Mean (SD) Age 21.4 (5.0) Years, SEARCH for Diabetes in Youth Study, 2014–2019. The SEARCH for Diabetes in Youth Study included 519 youth with type 2 diabetes (more...)
Medications for Other Health Conditions
Unlike adults with type 2 diabetes, youth with type 2 diabetes face a high burden of diabetic complications following a relatively short disease duration (158). Examples of comorbidities and complications that may require medications include dyslipidemia, nephropathy, hypertension, neuropathy, nonalcoholic fatty liver disease, polycystic ovary syndrome, and mental health concerns. The prevalence of use of medications for these conditions in clinical practice is not well described. In SEARCH, at a mean age of 26.5 years and diabetes duration of 13.2 years, 8.8% of youth and young adults with type 2 diabetes were on statins and 1.1% were on fibrates; 15.9% were on ACE inhibitors or ARBs, and 2.7% were on other antihypertensive medications (169). These data reflect that many of these young adults are not treated or are inadequately treated, as a large percentage of them had hypertension and dyslipidemia.
Glucose Monitoring and Other Technologies
The use of technology, such as CGM, as a tool for improving diabetes management has not yet been widely explored in youth with type 2 diabetes (170). In addition to CGM, youth with uncontrolled type 2 diabetes who require multiple daily injection insulin treatment may benefit from insulin pumps and AID systems. The prevalence of technology use in youth with type 2 diabetes in the United States has not yet been reported.
Glucose self-monitoring guidelines for youth with type 2 diabetes are less clear compared to those for youth with type 1 diabetes. ADA recommendations on youth with type 2 diabetes state that “blood glucose monitoring should be individualized, taking into consideration the pharmacologic treatment of the patient” (54). Data from SEARCH in 2001–2005 demonstrate that glucose monitoring frequency varies by treatment regimen, with youth on insulin, with or without an oral antihyperglycemic agent, having a higher frequency of glucose monitoring than youth treated with one or more oral agent or with lifestyle modification (Figure 16) (171).
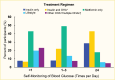
FIGURE 16.
Frequency of Self-Monitoring of Blood Glucose Among Youth Age 10–20 Years With Type 2 Diabetes, by Treatment Regimen, SEARCH for Diabetes in Youth Study, 2001–2005. OHA, oral hyperglycemic agent.
According to ADA guidelines, glycemic status should be assessed every 3 months with a goal A1C <7% for most children and adolescents with type 2 diabetes (54). A more stringent A1C target <6.5% may be appropriate, if it can be achieved without significant hypoglycemia or other adverse effects of treatment, and a less stringent A1C goal of <7.5% (<58 mmol/mol) may be appropriate, if there is increased risk of hypoglycemia (54).
Self-Care Practices
Youth with type 2 diabetes often have variable degrees of independence and autonomy regarding diabetes self-management, which evolve over time. In general, the age at diagnosis for youth with type 2 diabetes is after puberty. Thus, some degree of independence often exists even from the time of type 2 diabetes onset given this developmental stage. However, transition to fully independent diabetes self-care may be complicated by other simultaneous transitions in education, living situation, and employment that adolescents and young adults (AYAs) commonly experience.
AYAs with insufficient knowledge of their medical condition or insufficient awareness and implementation of optimal self-care practices are at risk for worsening of glycemic control. Thus, an organized and planned transition with education and psychosocial support starting from early adolescence is crucial to help AYAs achieve a successful transition to adult medical care, as mentioned in the Type 1 Diabetes in Youth: Self-Care Practices section. No transition program similar to LEAP for youth with type 1 diabetes has been reported for youth with type 2 diabetes (59).
Data from 182 youth and young adults with type 2 diabetes from SEARCH suggest that those who transferred to an adult (n=102) or to no (n=28) care provider had a higher likelihood of poor glycemic control at follow-up compared to those who remained with their pediatric provider (n=52) after adjustment for sex, age, race and ethnicity, or baseline A1C level (172). Possible explanations for the worsened glycemic control include gaps in medical care, loss of structural supports in family and school, and competing priorities that decrease diabetes self-care during the transition period.
It is imperative to not only address barriers to successful transition to adult care, but also to ensure that providers caring for individuals with youth-onset diabetes recognize the more aggressive nature of youth-onset type 2 diabetes compared to adult-onset type 2 diabetes. This difference remains a significant issue to address, particularly given the high risk of diabetes-related comorbidities.
Conclusion
In conclusion, in the past two decades, many new medications and technologies have emerged that can be used in the management of type 1 and type 2 diabetes. Technology options and use in adults and youth with diabetes, such as CGMs, insulin pumps, and AID systems, have continued to advance and become more widely used in diabetes management. The use of more recently approved medications in clinical practice has increased, particularly in adults with type 2 diabetes, and will continue to expand as new medication therapies are developed. Studies are underway to assess the use of GLP-1 RAs, SGLT2 inhibitors, and finerenone in type 1 diabetes, particularly for those with preexisting renal disease. A major challenge will be to provide equitable access to newer therapies and technologies, as well as DSMES, so that all can benefit from these treatments, leading to improved outcomes for all individuals with diabetes in the United States.
List of Abbreviations
- A1C
glycated hemoglobin
- ACE
angiotensin-converting enzyme
- ADA
American Diabetes Association
- AID
automated insulin delivery
- ARB
angiotensin II receptor blocker
- ASCVD
atherosclerotic cardiovascular disease
- AYA
adolescents and young adults
- BMI
body mass index
- CGM
continuous glucose monitor
- DPP-4
dipeptidyl peptidase-4
- DSMES
diabetes self-management and education support
- FDA
U.S. Food and Drug Administration
- FPG
fasting plasma glucose
- GLP-1 RA
glucagon-like peptide-1 receptor agonist
- ICD10
International Classification of Diseases, Tenth Revision
- LEAP
Let’s Empower and Prepare program
- NHANES
National Health and Nutrition Examination Survey
- NHIS
National Health Interview Survey
- RCT
randomized controlled trial
- SEARCH
SEARCH for Diabetes in Youth study
- SGLT2
sodium-glucose cotransporter-2
- TODAY
Treatment Options for Type 2 Diabetes in Adolescents and Youth study
Conversions
A1C: (% x 10.93) - 23.50 = mmol/mol
Glucose: mg/dL x 0.0555 = mmol/L
Acknowledgment
This is an update of: Saydah SH: Medication Use and Self-Care Practices in Persons With Diabetes. Chapter 39 in Diabetes in America, 3rd ed. Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, Knowler WC, Barrett-Connor E, Becker DJ, Brancati FL, Boyko EJ, Herman WH, Howard BV, Narayan KMV, Rewers M, Fradkin JE, Eds. Bethesda, MD, National Institutes of Health, NIH Pub No. 17-1468, 2018, p. 39.1–39.14.
Article History
Received in final form on May 7, 2024.
References
- 1.
- Saydah SH. Medication Use and Self-Care Practices in Persons With Diabetes. In: Cowie CC, Casagrande SS, Menke A, et al, eds. Diabetes in America. 3rd ed. National Institute of Diabetes and Digestive and Kidney Diseases; 2018. https://www
.ncbi.nlm .nih.gov/books/NBK567996/ [PubMed: 33651554] - 2.
- Optum. Optum Clinformatics Data Mart. https://www
.optum.com /content/dam/optum/resources /productSheets /Clinformatics_for_Data_Mart.pdf - 3.
- Chua KP, Lee JM, Conti RM. Out-of-pocket spending for insulin, diabetes-related supplies, and other health care services among privately insured US patients with type 1 diabetes. JAMA Intern Med. 2020;180(7):1012-1014. doi:10.1001/jamainternmed.2020.1308 [PMC free article: PMC7265118] [PubMed: 32478819] [CrossRef]
- 4.
- Centers for Disease Control and Prevention. Vision and Eye Health Surveillance System. About the Data: MarketScan. Updated May 14, 2024. https://www
.cdc.gov/vision-health-data /data-sources /marketscan.html - 5.
- Hamman RF, Bell RA, Dabelea D, et al. The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care. 2014;37(12):3336-3344. doi:10.2337/dc14-0574 [PMC free article: PMC4237981] [PubMed: 25414389] [CrossRef]
- 6.
- Malik FS, Sauder KA, Isom S, et al. Trends in glycemic control among youth and young adults with diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care. 2022;45(2):285-294. doi:10.2337/dc21-0507 [PMC free article: PMC8914430] [PubMed: 34995346] [CrossRef]
- 7.
- Wagenknecht LE, Lawrence JM, Isom S, et al. Trends in incidence of youth-onset type 1 and type 2 diabetes in the USA, 2002–18: results from the population-based SEARCH for Diabetes in Youth study. Lancet Diabetes Endocrinol. 2023;11(4):242-250. doi:10.1016/S2213-8587(23)00025-6 [PMC free article: PMC10091237] [PubMed: 36868256] [CrossRef]
- 8.
- Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):66-72. doi:10.1089/dia.2018.0384 [PMC free article: PMC7061293] [PubMed: 30657336] [CrossRef]
- 9.
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey. Updated May 13, 2024. https://www
.cdc.gov/nchs/nhanes/index .htm - 10.
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Health Interview Survey. Updated May 16, 2024. https://www
.cdc.gov/nchs/nhis/index.htm - 11.
- Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long-term trends in antidiabetes drug usage in the U.S.: real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care. 2018;41(1):69-78. doi:10.2337/dc17-1414 [PubMed: 29109299] [CrossRef]
- 12.
- TODAY Study Group; Zeitler P, Hirst K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247-2256. doi:10.1056/NEJMoa1109333 [PMC free article: PMC3478667] [PubMed: 22540912] [CrossRef]
- 13.
- Mosslemi M, Park HL, McLaren CE, Wong ND. A treatment-based algorithm for identification of diabetes type in the National Health and Nutrition Examination Survey. Cardiovasc Endocrinol Metab. 2020;9(1):9-16. doi:10.1097/XCE.0000000000000189 [PMC free article: PMC7041875] [PubMed: 32104786] [CrossRef]
- 14.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Prevalence of Diagnosed Diabetes. Updated May 14, 2024. Accessed October 26, 2022, https://www
.cdc.gov/diabetes /php/data-research/ - 15.
- IBM. Welcome to MarketScan Research. Accessed October 26, 2022, https://www
.ibm.com/watson /health/resources/ipv-opv/ - 16.
- Sjöholm Å. Using adjuvant pharmacotherapy in the treatment of type 1 diabetes. Expert Opin Pharmacother. 2021;22(16):2143-2148. doi:10.1080/14656566.2021.1939679 [PubMed: 34132620] [CrossRef]
- 17.
- Wright LA, Hirsch IB. Non-insulin treatments for type 1 diabetes: critical appraisal of the available evidence and insight into future directions. Diabet Med. 2019;36(6):665-678. doi:10.1111/dme.13941 [PubMed: 30801765] [CrossRef]
- 18.
- Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Mortality in type 1 diabetes in the DCCT/EDIC versus the general population. Diabetes Care. 2016;39(8):1378-1383. doi:10.2337/dc15-2399 [PMC free article: PMC4955932] [PubMed: 27411699] [CrossRef]
- 19.
- Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371(21):1972-1982. doi:10.1056/NEJMoa1408214 [PubMed: 25409370] [CrossRef]
- 20.
- Costacou T, Orchard TJ. Cumulative kidney complication risk by 50 years of type 1 diabetes: the effects of sex, age, and calendar year at onset. Diabetes Care. 2018;41(3):426-433. doi:10.2337/dc17-1118 [PMC free article: PMC5829956] [PubMed: 28931542] [CrossRef]
- 21.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456-1462. doi:10.1056/NEJM199311113292004 [PubMed: 8413456] [CrossRef]
- 22.
- Chalakova T, Yotov Y, Tzotchev K, et al. Type 1 diabetes mellitus—risk factor for cardiovascular disease morbidity and mortality. Curr Diabetes Rev. 2021;17(1):37-54. doi:10.2174/1573399816666200511004205 [PubMed: 32389113] [CrossRef]
- 23.
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S144-S174. doi:10.2337/dc22-S010 [PubMed: 34964815] [CrossRef]
- 24.
- Hero C, Rawshani A, Svensson AM, et al. Association between use of lipid-lowering therapy and cardiovascular diseases and death in individuals with type 1 diabetes. Diabetes Care. 2016;39(6):996-1003. doi:10.2337/dc15-2450 [PubMed: 27208327] [CrossRef]
- 25.
- Carreira M, Ruiz de Adana MS, Pinzon JL, Anarte-Ortiz MT. Internet-based cognitive-behavioral therapy (CBT) for depressive symptomatology in individuals with type 1 diabetes (WEB_TDDI1 study): a randomized controlled trial protocol. PLoS One. 2022;17(9):e0274551. doi:10.1371/journal.pone.0274551 [PMC free article: PMC9488778] [PubMed: 36126050] [CrossRef]
- 26.
- Ebekozien O, Mungmode A, Sanchez J, et al. Longitudinal trends in glycemic outcomes and technology use for over 48,000 people with type 1 diabetes (2016–2022) from the T1D Exchange Quality Improvement Collaborative. Diabetes Technol Ther. 2023;25(11):765-773. doi:10.1089/dia.2023.0320 [PubMed: 37768677] [CrossRef]
- 27.
- DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D Exchange real-world observational study. J Diabetes Sci Technol. 2023;17(2):322-328. doi:10.1177/19322968211049783 [PMC free article: PMC10012384] [PubMed: 34632823] [CrossRef]
- 28.
- Zylke JW, DeAngelis CD. Pediatric chronic diseases—stealing childhood. JAMA. 2007;297(24):2765-2766. doi:10.1001/jama.297.24.2765 [PubMed: 17595280] [CrossRef]
- 29.
- Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths—selected counties and Indian reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep. 2020;69(6):161-165. doi:10.15585/mmwr.mm6906a3 [PMC free article: PMC7017961] [PubMed: 32053581] [CrossRef]
- 30.
- Lawrence JM, Divers J, Isom S, et al. Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001–2017. JAMA. 2021;326(8):717-727. doi:10.1001/jama.2021.11165 [PMC free article: PMC8385600] [PubMed: 34427600] [CrossRef]
- 31.
- Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317(8):825-835. doi:10.1001/jama.2017.0686 [PMC free article: PMC5483855] [PubMed: 28245334] [CrossRef]
- 32.
- Everett EM, Copeland TP, Moin T, Wisk LE. National trends in pediatric admissions for diabetic ketoacidosis, 2006–2016. J Clin Endocrinol Metab. 2021;106(8):2343-2354. doi:10.1210/clinem/dgab287 [PMC free article: PMC8277205] [PubMed: 33942077] [CrossRef]
- 33.
- Everett EM, Copeland TP, Moin T, Wisk LE. Insulin pump-related inpatient admissions in a national sample of youth with type 1 diabetes. J Clin Endocrinol Metab. 2022;107(6):e2381-e2387. doi:10.1210/clinem/dgac047 [PMC free article: PMC9113825] [PubMed: 35196382] [CrossRef]
- 34.
- Addala A, Auzanneau M, Miller K, et al. A decade of disparities in diabetes technology use and HbA(1c) in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care. 2021;44(1):133-140. doi:10.2337/dc20-0257 [PMC free article: PMC8162452] [PubMed: 32938745] [CrossRef]
- 35.
- Everett EM, Wright D, Williams A, et al. A longitudinal view of disparities in insulin pump use among youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Technol Ther. 2023;25(2):131-139. doi:10.1089/dia.2022.0340 [PMC free article: PMC9894603] [PubMed: 36475821] [CrossRef]
- 36.
- Aberer F, Pieber TR, Eckstein ML, Sourij H, Moser O. Glucose-lowering therapy beyond insulin in type 1 diabetes: a narrative review on existing evidence from randomized controlled trials and clinical perspective. Pharmaceutics. 2022;14(6):1180. doi:10.3390/pharmaceutics14061180 [PMC free article: PMC9229408] [PubMed: 35745754] [CrossRef]
- 37.
- Liu Y, Chen H, Li H, Li L, Wu J, Li H. Effect and safety of adding metformin to insulin therapy in treating adolescents with type 1 diabetes mellitus: an updated meta-analysis of 10 randomized controlled trials. Front Endocrinol (Lausanne). 2022;13:878585. doi:10.3389/fendo.2022.878585 [PMC free article: PMC9190285] [PubMed: 35707462] [CrossRef]
- 38.
- Galderisi A, Sherr J, VanName M, et al. Pramlintide but not liraglutide suppresses meal-stimulated glucagon responses in type 1 diabetes. J Clin Endocrinol Metab. 2018;103(3):1088-1094. doi:10.1210/jc.2017-02265 [PMC free article: PMC6276715] [PubMed: 29211871] [CrossRef]
- 39.
- Wood JR, Silverstein J. Incretins and amylin in pediatric diabetes: new tools for management of diabetes in youth. Curr Opin Pediatr. 2013;25(4):502-508. doi:10.1097/MOP.0b013e328362fdfb [PubMed: 23782574] [CrossRef]
- 40.
- Drozd I, Weiskorn J, Lange K, et al. Prevalence of LDL-hypercholesterolemia and other cardiovascular risk factors in young people with type 1 diabetes. J Clin Lipidol. 2023;17(4):483-490. doi:10.1016/j.jacl.2023.05.097 [PubMed: 37258406] [CrossRef]
- 41.
- Garey CJ, Clements MA, McAuliffe-Fogarty AH, et al. The association between depression symptom endorsement and glycemic outcomes in adolescents with type 1 diabetes. Pediatr Diabetes. 2022;23(2):248-257. doi:10.1111/pedi.13290 [PubMed: 34779100] [CrossRef]
- 42.
- Nip ASY, Reboussin BA, Dabelea D, et al. Disordered eating behaviors in youth and young adults with type 1 or type 2 diabetes receiving insulin therapy: the SEARCH for Diabetes in Youth study. Diabetes Care. 2019;42(5):859-866. doi:10.2337/dc18-2420 [PMC free article: PMC6489106] [PubMed: 30862656] [CrossRef]
- 43.
- Rechenberg K, Whittemore R, Grey M. Anxiety in youth with type 1 diabetes. J Pediatr Nurs. 2017;32:64-71. doi:10.1016/j.pedn.2016.08.007 [PMC free article: PMC5743322] [PubMed: 27663096] [CrossRef]
- 44.
- Jackson S, Creo A, Kumar S. Are clinicians aggressive enough in treating diabetes-related hyperlipidemia in youth? Curr Atheroscler Rep. 2022;24(6):471-481. doi:10.1007/s11883-022-01020-y [PubMed: 35404039] [CrossRef]
- 45.
- Katz ML, Guo Z, Laffel LM. Management of hypertension and high low-density lipoprotein in pediatric type 1 diabetes. J Pediatr. 2018;197:140-146 e12. doi:10.1016/j.jpeds.2017.11.059 [PMC free article: PMC6013061] [PubMed: 29395184] [CrossRef]
- 46.
- Hirsch IB, Miller E. Integrating continuous glucose monitoring into clinical practices and patients’ lives. Diabetes Technol Ther. 2021;23(S3):S72-S80. doi:10.1089/dia.2021.0233 [PubMed: 34546085] [CrossRef]
- 47.
- Galindo RJ, Aleppo G. Continuous glucose monitoring: the achievement of 100 years of innovation in diabetes technology. Diabetes Res Clin Pract. 2020;170:108502. doi:10.1016/j.diabres.2020.108502 [PMC free article: PMC7736459] [PubMed: 33065179] [CrossRef]
- 48.
- Beck RW, Bergenstal RM, Laffel LM, Pickup JC. Advances in technology for management of type 1 diabetes. Lancet. 2019;394(10205):1265-1273. doi:10.1016/S0140-6736(19)31142-0 [PubMed: 31533908] [CrossRef]
- 49.
- Sawyer A, Sobczak M, Forlenza GP, Alonso GT. Glycemic control in relation to technology use in a single-center cohort of children with type 1 diabetes. Diabetes Technol Ther. 2022;24(6):409-415. doi:10.1089/dia.2021.0471 [PMC free article: PMC9208858] [PubMed: 35099306] [CrossRef]
- 50.
- Abraham MB, de Bock M, Smith GJ, et al. Effect of a hybrid closed-loop system on glycemic and psychosocial outcomes in children and adolescents with type 1 diabetes: a randomized clinical trial. JAMA Pediatr. 2021;175(12):1227-1235. doi:10.1001/jamapediatrics.2021.3965 [PMC free article: PMC8506294] [PubMed: 34633418] [CrossRef]
- 51.
- Ware J, Allen JM, Boughton CK, et al. Randomized trial of closed-loop control in very young children with type 1 diabetes. N Engl J Med. 2022;386(3):209-219. doi:10.1056/NEJMoa2111673 [PubMed: 35045227] [CrossRef]
- 52.
- Wadwa RP, Reed ZW, Buckingham BA, et al. Trial of hybrid closed-loop control in young children with type 1 diabetes. N Engl J Med. 2023;388(11):991-1001. doi:10.1056/NEJMoa2210834 [PMC free article: PMC10082994] [PubMed: 36920756] [CrossRef]
- 53.
- Levine BS, Anderson BJ, Butler DA, Antisdel JE, Brackett J, Laffel LM. Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. J Pediatr. 2001;139(2):197-203. doi:10.1067/mpd.2001.116283 [PubMed: 11487743] [CrossRef]
- 54.
- American Diabetes Association Professional Practice Committee. 14. Children and Adolescents: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S258-S281. doi:10.2337/dc24-S014 [PMC free article: PMC10725814] [PubMed: 38078582] [CrossRef]
- 55.
- Miller KM, Beck RW, Bergenstal RM, et al. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D Exchange clinic registry participants. Diabetes Care. 2013;36(7):2009-2014. doi:10.2337/dc12-1770 [PMC free article: PMC3687326] [PubMed: 23378621] [CrossRef]
- 56.
- Reid LA, Geraci M, Mendoza JA, et al. Household food insecurity is associated with physical activity in youth and young adults with diabetes: a cross-sectional study. J Phys Act Health. 2023;21(1):77-84. doi:10.1123/jpah.2022-0338 [PubMed: 37922896] [CrossRef]
- 57.
- Montali L, Zulato E, Cornara M, Ausili D, Luciani M. Barriers and facilitators of type 1 diabetes self-care in adolescents and young adults. J Pediatr Nurs. 2022;62:136-143. doi:10.1016/j.pedn.2021.09.014 [PubMed: 34561133] [CrossRef]
- 58.
- Helgeson VS. Diabetes burnout among emerging adults with type 1 diabetes: a mixed methods investigation. J Behav Med. 2021;44(3):368-378. doi:10.1007/s10865-020-00198-3 [PubMed: 33566266] [CrossRef]
- 59.
- Sequeira PA, Pyatak EA, Weigensberg MJ, et al. Let’s Empower and Prepare (LEAP): evaluation of a structured transition program for young adults with type 1 diabetes. Diabetes Care. 2015;38(8):1412-1419. doi:10.2337/dc14-2577 [PMC free article: PMC4512132] [PubMed: 25906787] [CrossRef]
- 60.
- Centers for Disease Control and Prevention. Diabetes Report Card 2021. Updated July 19, 2022. https://archive
.cdc.gov /www_cdc_gov/diabetes /library/reports/reportcard.html - 61.
- American Diabetes Association. Standards of Care in Diabetes-2023 abridged for primary care providers. Clin Diabetes. 2022;41(1):4-31. doi:10.2337/cd23-as01 [PMC free article: PMC9845083] [PubMed: 36714254] [CrossRef]
- 62.
- Le P, Chaitoff A, Misra-Hebert AD, Ye W, Herman WH, Rothberg MB. Use of antihyperglycemic medications in U.S. adults: an analysis of the National Health and Nutrition Examination Survey. Diabetes Care. 2020;43(6):1227-1233. doi:10.2337/dc19-2424 [PubMed: 32234720] [CrossRef]
- 63.
- Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999–2018. N Engl J Med. 2021;384(23):2219-2228. doi:10.1056/NEJMsa2032271 [PMC free article: PMC8385648] [PubMed: 34107181] [CrossRef]
- 64.
- Shin H, Schneeweiss S, Glynn RJ, Patorno E. Trends in first-line glucose-lowering drug use in adults with type 2 diabetes in light of emerging evidence for SGLT-2i and GLP-1RA. Diabetes Care. 2021;44(8):1774-1782. doi:10.2337/dc20-2926 [PMC free article: PMC8385465] [PubMed: 34385345] [CrossRef]
- 65.
- Landon BE, Zaslavsky AM, Souza J, Ayanian JZ. Trends in diabetes treatment and monitoring among Medicare beneficiaries. J Gen Intern Med. 2018;33(4):471-480. doi:10.1007/s11606-018-4310-4 [PMC free article: PMC5880782] [PubMed: 29427177] [CrossRef]
- 66.
- Sarkar S, Heyward J, Alexander GC, Kalyani RR. Trends in insulin types and devices used by adults with type 2 diabetes in the United States, 2016 to 2020. JAMA Netw Open. 2021;4(10):e2128782. doi:10.1001/jamanetworkopen.2021.28782 [PMC free article: PMC8511976] [PubMed: 34636912] [CrossRef]
- 67.
- Ding D, Glied SA. Disparities in the Use of New Diabetes Medications: Widening Treatment Inequality by Race and Insurance Coverage. The Commonwealth Fund; June 2022. https://www
.commonwealthfund .org/publications /issue-briefs/2022 /jun/disparities-use-new-diabetes-medications-treatment-inequality. doi:10.26099/vabp-0g69 [CrossRef] - 68.
- Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137-188. doi:10.1152/physrev.00045.2011 [PubMed: 23303908] [CrossRef]
- 69.
- Iglay K, Hannachi H, Howie PJ, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(7):1243-1252. doi:10.1185/03007995.2016.1168291 [PubMed: 26986190] [CrossRef]
- 70.
- Naha S, Gardner MJ, Khangura D, Kurukulasuriya LR, Sowers JR. Hypertension in Diabetes. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext [Internet]. eBook. MDText.com, Inc.; 2000. https://www
.ncbi.nlm .nih.gov/books/NBK279027/ - 71.
- Pantalone KM, Hobbs TM, Wells BJ, et al. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res Care. 2015;3(1):e000093. doi:10.1136/bmjdrc-2015-000093 [PMC free article: PMC4513350] [PubMed: 26217493] [CrossRef]
- 72.
- Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717. doi:10.1136/bmj.i717 [PMC free article: PMC4770818] [PubMed: 26920333] [CrossRef]
- 73.
- Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615. doi:10.1001/jama.2014.18574 [PubMed: 25668264] [CrossRef]
- 74.
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957-967. doi:10.1016/S0140-6736(15)01225-8 [PubMed: 26724178] [CrossRef]
- 75.
- Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851-860. doi:10.1056/NEJMoa011303 [PubMed: 11565517] [CrossRef]
- 76.
- Barzilay JI, Davis BR, Bettencourt J, et al. Cardiovascular outcomes using doxazosin vs. chlorthalidone for the treatment of hypertension in older adults with and without glucose disorders: a report from the ALLHAT study. J Clin Hypertens (Greenwich). 2004;6(3):116-125. doi:10.1111/j.1524-6175.2004.03216.x [PMC free article: PMC8109632] [PubMed: 15010644] [CrossRef]
- 77.
- Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385(9982):2047-2056. doi:10.1016/S0140-6736(14)62459-4 [PubMed: 26009228] [CrossRef]
- 78.
- Weber MA, Bakris GL, Jamerson K, et al. Cardiovascular events during differing hypertension therapies in patients with diabetes. J Am Coll Cardiol. 2010;56(1):77-85. doi:10.1016/j.jacc.2010.02.046 [PubMed: 20620720] [CrossRef]
- 79.
- Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971. doi:10.1371/journal.pmed.1001971 [PMC free article: PMC4783064] [PubMed: 26954482] [CrossRef]
- 80.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985-3023. doi:10.1016/j.jacc.2013.11.004 [PubMed: 24239920] [CrossRef]
- 81.
- Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. doi:10.1056/NEJMoa1800389 [PubMed: 29897866] [CrossRef]
- 82.
- Sever PS, Poulter NR, Dahlof B, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial—lipid-lowering arm (ASCOT-LLA). Diabetes Care. 2005;28(5):1151-1157. doi:10.2337/diacare.28.5.1151 [PubMed: 15855581] [CrossRef]
- 83.
- Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29(6):1220-1226. doi:10.2337/dc05-2465 [PubMed: 16731999] [CrossRef]
- 84.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685-696. doi:10.1016/S0140-6736(04)16895-5 [PubMed: 15325833] [CrossRef]
- 85.
- Knopp RH, d’Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29(7):1478-1485. doi:10.2337/dc05-2415 [PubMed: 16801565] [CrossRef]
- 86.
- Cholesterol Treatment Trialists Collaborators; Kearney PM, Blackwell L, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117-125. doi:10.1016/S0140-6736(08)60104-X [PubMed: 18191683] [CrossRef]
- 87.
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677 [PMC free article: PMC8351755] [PubMed: 30879339] [CrossRef]
- 88.
- Antithrombotic Trialists Collaboration; Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849-1860. doi:10.1016/S0140-6736(09)60503-1 [PMC free article: PMC2715005] [PubMed: 19482214] [CrossRef]
- 89.
- ASCEND Study Collaborative Group; Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988 [PubMed: 30146931] [CrossRef]
- 90.
- Belch J, MacCuish A, Campbell I, et al. The Prevention of Progression of Arterial Disease and Diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. doi:10.1136/bmj.a1840 [PMC free article: PMC2658865] [PubMed: 18927173] [CrossRef]
- 91.
- De Berardis G, Sacco M, Strippoli GF, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ. 2009;339:b4531. doi:10.1136/bmj.b4531 [PMC free article: PMC2774388] [PubMed: 19897665] [CrossRef]
- 92.
- McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819 [PMC free article: PMC6289056] [PubMed: 30221597] [CrossRef]
- 93.
- Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008;51(3):408-416. doi:10.1007/s00125-007-0911-x [PMC free article: PMC2235909] [PubMed: 18204830] [CrossRef]
- 94.
- Farmer A, Wade A, Goyder E, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335(7611):132. doi:10.1136/bmj.39247.447431.BE [PMC free article: PMC1925177] [PubMed: 17591623] [CrossRef]
- 95.
- Young LA, Buse JB, Weaver MA, et al. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017;177(7):920-929. doi:10.1001/jamainternmed.2017.1233 [PMC free article: PMC5818811] [PubMed: 28600913] [CrossRef]
- 96.
- Mannucci E, Antenore A, Giorgino F, Scavini M. Effects of structured versus unstructured self-monitoring of blood glucose on glucose control in patients with non-insulin-treated type 2 diabetes: a meta-analysis of randomized controlled trials. J Diabetes Sci Technol. 2018;12(1):183-189. doi:10.1177/1932296817719290 [PMC free article: PMC5761981] [PubMed: 28697625] [CrossRef]
- 97.
- Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262-2272. doi:10.1001/jama.2021.7444 [PMC free article: PMC8173473] [PubMed: 34077499] [CrossRef]
- 98.
- Ehrhardt NM, Chellappa M, Walker MS, Fonda SJ, Vigersky RA. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5(3):668-675. doi:10.1177/193229681100500320 [PMC free article: PMC3192632] [PubMed: 21722581] [CrossRef]
- 99.
- Ida S, Kaneko R, Murata K. Utility of real-time and retrospective continuous glucose monitoring in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. J Diabetes Res. 2019;2019:4684815. doi:10.1155/2019/4684815 [PMC free article: PMC6350576] [PubMed: 30775385] [CrossRef]
- 100.
- Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365-374. doi:10.7326/M16-2855 [PubMed: 28828487] [CrossRef]
- 101.
- Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55-73. doi:10.1007/s13300-016-0223-6 [PMC free article: PMC5306122] [PubMed: 28000140] [CrossRef]
- 102.
- Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42(7):1178-1184. doi:10.2337/dc18-0166 [PubMed: 31036546] [CrossRef]
- 103.
- Wada E, Onoue T, Kobayashi T, et al. Flash glucose monitoring helps achieve better glycemic control than conventional self-monitoring of blood glucose in non-insulin-treated type 2 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care. 2020;8(1):e001115. doi:10.1136/bmjdrc-2019-001115 [PMC free article: PMC7292039] [PubMed: 32518063] [CrossRef]
- 104.
- Elliott T, Beca S, Beharry R, Tsoukas MA, Zarruk A, Abitbol A. The impact of flash glucose monitoring on glycated hemoglobin in type 2 diabetes managed with basal insulin in Canada: a retrospective real-world chart review study. Diab Vasc Dis Res. 2021;18(4). doi:10.1177/14791641211021374 [PMC free article: PMC8481728] [PubMed: 34275385] [CrossRef]
- 105.
- Wright EE, Jr., Kerr MSD, Reyes IJ, Nabutovsky Y, Miller E. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34(2):184-189. doi:10.2337/ds20-0069 [PMC free article: PMC8178717] [PubMed: 34149259] [CrossRef]
- 106.
- Puckrein GA, Hirsch IB, Parkin CG, et al. Assessment of glucose monitoring adherence in Medicare beneficiaries with insulin-treated diabetes. Diabetes Technol Ther. 2023;25(1):31-38. doi:10.1089/dia.2022.0377 [PubMed: 36409474] [CrossRef]
- 107.
- American Diabetes Association Professional Practice Committee. 1. Improving Care and Promoting Health in Populations: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S8-S16. doi:10.2337/dc22-S001 [PubMed: 34964872] [CrossRef]
- 108.
- Powers MA, Bardsley JK, Cypress M, et al. Diabetes Self-management Education and Support in Adults With Type 2 Diabetes: a consensus report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. Diabetes Care. 2020;43(7):1636-1649. doi:10.2337/dci20-0023 [PubMed: 32513817] [CrossRef]
- 109.
- Liu G, Li Y, Pan A, et al. Adherence to a healthy lifestyle in association with microvascular complications among adults with type 2 diabetes. JAMA Netw Open. 2023;6(1):e2252239. doi:10.1001/jamanetworkopen.2022.52239 [PMC free article: PMC9880795] [PubMed: 36701156] [CrossRef]
- 110.
- Mendez I, Lundeen EA, Saunders M, Williams A, Saaddine J, Albright A. Diabetes self-management education and association with diabetes self-care and clinical preventive care practices. Sci Diabetes Self Manag Care. 2022;48(1):23-34. doi:10.1177/26350106211065378 [PMC free article: PMC10979825] [PubMed: 35023406] [CrossRef]
- 111.
- Centers for Disease Control and Prevention. US Diabetes Surveillance System (USDSS). Accessed December 2, 2022. https://gis
.cdc.gov/grasp /diabetes/diabetesatlas-surveillance.html# - 112.
- Fang M. Trends in diabetes management among US adults: 1999–2016. J Gen Intern Med. 2020;35(5):1427-1434. doi:10.1007/s11606-019-05587-2 [PMC free article: PMC7210372] [PubMed: 31898135] [CrossRef]
- 113.
- Duncan I, Ahmed T, Li QE, et al. Assessing the value of the diabetes educator. Diabetes Educ. 2011;37(5):638-657. doi:10.1177/0145721711416256 [PubMed: 21878591] [CrossRef]
- 114.
- Horigan G, Davies M, Findlay-White F, Chaney D, Coates V. Reasons why patients referred to diabetes education programmes choose not to attend: a systematic review. Diabet Med. 2017;34(1):14-26. doi:10.1111/dme.13120 [PubMed: 26996982] [CrossRef]
- 115.
- Carey ME, Agarwal S, Horne R, Davies M, Slevin M, Coates V. Exploring organizational support for the provision of structured self-management education for people with type 2 diabetes: findings from a qualitative study. Diabet Med. 2019;36(6):761-770. doi:10.1111/dme.13946 [PubMed: 30868654] [CrossRef]
- 116.
- Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731-754. doi:10.2337/dci19-0014 [PMC free article: PMC7011201] [PubMed: 31000505] [CrossRef]
- 117.
- American Diabetes Association Professional Practice Committee. 5. Facilitating Behavior Change and Well-Being to Improve Health Outcomes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S60-S82. doi:10.2337/dc22-S005 [PubMed: 34964866] [CrossRef]
- 118.
- National Clinical Care Commission. Report to Congress on Leveraging Federal Programs to Prevent and Control Diabetes and Its Complications. 2021. https://health
.gov/sites /default/files/2022-01 /NCCC%20Report%20to%20Congress.pdf - 119.
- Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140. doi:10.2337/dc16-2053 [PMC free article: PMC5127231] [PubMed: 27879358] [CrossRef]
- 120.
- Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Barriers to diabetes management: patient and provider factors. Diabetes Res Clin Pract. 2011;93(1):1-9. doi:10.1016/j.diabres.2011.02.002 [PubMed: 21382643] [CrossRef]
- 121.
- Sarkar U, Fisher L, Schillinger D. Is self-efficacy associated with diabetes self-management across race/ethnicity and health literacy? Diabetes Care. 2006;29(4):823-829. doi:10.2337/diacare.29.04.06.dc05-1615 [PubMed: 16567822] [CrossRef]
- 122.
- King DK, Glasgow RE, Toobert DJ, et al. Self-efficacy, problem solving, and social-environmental support are associated with diabetes self-management behaviors. Diabetes Care. 2010;33(4):751-753. doi:10.2337/dc09-1746 [PMC free article: PMC2845021] [PubMed: 20150299] [CrossRef]
- 123.
- Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008;31(12):2398-2403. doi:10.2337/dc08-1341 [PMC free article: PMC2584202] [PubMed: 19033420] [CrossRef]
- 124.
- Mulligan K, McBain H, Lamontagne-Godwin F, et al. Barriers to effective diabetes management—a survey of people with severe mental illness. BMC Psychiatry. 2018;18(1):165. doi:10.1186/s12888-018-1744-5 [PMC free article: PMC5984777] [PubMed: 29859061] [CrossRef]
- 125.
- Gary TL, Safford MM, Gerzoff RB, et al. Perception of neighborhood problems, health behaviors, and diabetes outcomes among adults with diabetes in managed care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 2008;31(2):273-278. doi:10.2337/dc07-1111 [PubMed: 18000180] [CrossRef]
- 126.
- Friis AM, Johnson MH, Cutfield RG, Consedine NS. Kindness matters: a randomized controlled trial of a mindful self-compassion intervention improves depression, distress, and HbA1c among patients with diabetes. Diabetes Care. 2016;39(11):1963-1971. doi:10.2337/dc16-0416 [PubMed: 27335319] [CrossRef]
- 127.
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140-S157. doi:10.2337/dc23-S009 [PMC free article: PMC9810476] [PubMed: 36507650] [CrossRef]
- 128.
- Lin LK, Sun Y, Heng BH, Chew DEK, Chong PN. Medication adherence and glycemic control among newly diagnosed diabetes patients. BMJ Open Diabetes Res Care. 2017;5(1):e000429. doi:10.1136/bmjdrc-2017-000429 [PMC free article: PMC5574459] [PubMed: 28878942] [CrossRef]
- 129.
- Khayyat SM, Mohamed MMA, Khayyat SMS, et al. Association between medication adherence and quality of life of patients with diabetes and hypertension attending primary care clinics: a cross-sectional survey. Qual Life Res. 2019;28(4):1053-1061. doi:10.1007/s11136-018-2060-8 [PubMed: 30470970] [CrossRef]
- 130.
- Egede LE, Gebregziabher M, Echols C, Lynch CP. Longitudinal effects of medication nonadherence on glycemic control. Ann Pharmacother. 2014;48(5):562-570. doi:10.1177/1060028014526362 [PubMed: 24586059] [CrossRef]
- 131.
- Patel S, Abreu M, Tumyan A, Adams-Huet B, Li X, Lingvay I. Effect of medication adherence on clinical outcomes in type 2 diabetes: analysis of the SIMPLE study. BMJ Open Diabetes Res Care. 2019;7(1):e000761. doi:10.1136/bmjdrc-2019-000761 [PMC free article: PMC6887507] [PubMed: 31803482] [CrossRef]
- 132.
- Egede LE, Gebregziabher M, Dismuke CE, et al. Medication nonadherence in diabetes: longitudinal effects on costs and potential cost savings from improvement. Diabetes Care. 2012;35(12):2533-2539. doi:10.2337/dc12-0572 [PMC free article: PMC3507586] [PubMed: 22912429] [CrossRef]
- 133.
- Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff (Millwood). 2011;30(1):91-99. doi:10.1377/hlthaff.2009.1087 [PubMed: 21209444] [CrossRef]
- 134.
- Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836-1841. doi:10.1001/archinte.166.17.1836 [PubMed: 17000939] [CrossRef]
- 135.
- Currie CJ, Peyrot M, Morgan CL, et al. The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care. 2012;35(6):1279-1284. doi:10.2337/dc11-1277 [PMC free article: PMC3357221] [PubMed: 22511257] [CrossRef]
- 136.
- Jha AK, Aubert RE, Yao J, Teagarden JR, Epstein RS. Greater adherence to diabetes drugs is linked to less hospital use and could save nearly $5 billion annually. Health Aff (Millwood). 2012;31(8):1836-1846. doi:10.1377/hlthaff.2011.1198 [PubMed: 22869663] [CrossRef]
- 137.
- Giugliano D, Maiorino MI, Bellastella G, Esposito K. Glycemic control in type 2 diabetes: from medication nonadherence to residual vascular risk. Endocrine. 2018;61(1):23-27. doi:10.1007/s12020-017-1517-9 [PubMed: 29322300] [CrossRef]
- 138.
- Chandran A, Bonafede MK, Nigam S, Saltiel-Berzin R, Hirsch LJ, Lahue BJ. Adherence to insulin pen therapy is associated with reduction in healthcare costs among patients with type 2 diabetes mellitus. Am Health Drug Benefits. 2015;8(3):148-158. [PMC free article: PMC4467016] [PubMed: 26085903]
- 139.
- Pawaskar MD, Camacho FT, Anderson RT, Cobden D, Joshi AV, Balkrishnan R. Health care costs and medication adherence associated with initiation of insulin pen therapy in Medicaid-enrolled patients with type 2 diabetes: a retrospective database analysis. Clin Ther. 2007;29(6 Pt 1):1294-1305. doi:10.1016/j.clinthera.2007.07.007 [PubMed: 18036391] [CrossRef]
- 140.
- Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care. 2013;51(8 Suppl 3):S11-S21. doi:10.1097/MLR.0b013e31829b1d2a [PMC free article: PMC3727405] [PubMed: 23774515] [CrossRef]
- 141.
- Farr AM, Sheehan JJ, Curkendall SM, Smith DM, Johnston SS, Kalsekar I. Retrospective analysis of long-term adherence to and persistence with DPP-4 inhibitors in US adults with type 2 diabetes mellitus. Adv Ther. 2014;31(12):1287-1305. doi:10.1007/s12325-014-0171-3 [PMC free article: PMC4271133] [PubMed: 25504156] [CrossRef]
- 142.
- Iglay K, Cartier SE, Rosen VM, et al. Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin. 2015;31(7):1283-1296. doi:10.1185/03007995.2015.1053048 [PubMed: 26023805] [CrossRef]
- 143.
- Perez-Nieves M, Boye KS, Kiljanski J, Cao D, Lage MJ. Adherence to basal insulin therapy among people with type 2 diabetes: a retrospective cohort study of costs and patient outcomes. Diabetes Ther. 2018;9(3):1099-1111. doi:10.1007/s13300-018-0421-5 [PMC free article: PMC5984924] [PubMed: 29644618] [CrossRef]
- 144.
- Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010;33(2):240-245. doi:10.2337/dc09-1348 [PMC free article: PMC2809256] [PubMed: 20103556] [CrossRef]
- 145.
- Kirkman MS, Rowan-Martin MT, Levin R, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care. 2015;38(4):604-609. doi:10.2337/dc14-2098 [PMC free article: PMC4370331] [PubMed: 25573883] [CrossRef]
- 146.
- Herkert D, Vijayakumar P, Luo J, et al. Cost-related insulin underuse among patients with diabetes. JAMA Intern Med. 2019;179(1):112-114. doi:10.1001/jamainternmed.2018.5008 [PMC free article: PMC6583414] [PubMed: 30508012] [CrossRef]
- 147.
- Taylor SI. The high cost of diabetes drugs: disparate impact on the most vulnerable patients. Diabetes Care. 2020;43(10):2330-2332. doi:10.2337/dci20-0039 [PMC free article: PMC8051261] [PubMed: 32958616] [CrossRef]
- 148.
- Patel MR, Piette JD, Resnicow K, Kowalski-Dobson T, Heisler M. Social determinants of health, cost-related nonadherence, and cost-reducing behaviors among adults with diabetes: findings from the National Health Interview Survey. Med Care. 2016;54(8):796-803. doi:10.1097/MLR.0000000000000565 [PMC free article: PMC4945373] [PubMed: 27219636] [CrossRef]
- 149.
- Cefalu WT, Dawes DE, Gavlak G, et al. Insulin Access and Affordability Working Group: conclusions and recommendations. Diabetes Care. 2018;41(6):1299-1311. doi:10.2337/dci18-0019 [PubMed: 29739814] [CrossRef]
- 150.
- Silverman J, Krieger J, Kiefer M, Hebert P, Robinson J, Nelson K. The relationship between food insecurity and depression, diabetes distress and medication adherence among low-income patients with poorly-controlled diabetes. J Gen Intern Med. 2015;30(10):1476-1480. doi:10.1007/s11606-015-3351-1 [PMC free article: PMC4579205] [PubMed: 25917659] [CrossRef]
- 151.
- Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44(1):258-279. doi:10.2337/dci20-0053 [PMC free article: PMC7783927] [PubMed: 33139407] [CrossRef]
- 152.
- Osborn CY, Cavanaugh K, Wallston KA, et al. Health literacy explains racial disparities in diabetes medication adherence. J Health Commun. 2011;16(Suppl 3):268-278. doi:10.1080/10810730.2011.604388 [PMC free article: PMC3561717] [PubMed: 21951257] [CrossRef]
- 153.
- Kang H, Lobo JM, Kim S, Sohn MW. Cost-related medication non-adherence among U.S. adults with diabetes. Diabetes Res Clin Pract. 2018;143:24-33. doi:10.1016/j.diabres.2018.06.016 [PMC free article: PMC6204232] [PubMed: 29944967] [CrossRef]
- 154.
- Slabaugh SL, Bouchard JR, Li Y, Baltz JC, Meah YA, Moretz DC. Characteristics relating to adherence and persistence to basal insulin regimens among elderly insulin-naïve patients with type 2 diabetes: pre-filled pens versus vials/syringes. Adv Ther. 2015;32(12):1206-1221. doi:10.1007/s12325-015-0266-5 [PMC free article: PMC4679781] [PubMed: 26563324] [CrossRef]
- 155.
- Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299-1307. doi:10.2147/PPA.S106821 [PMC free article: PMC4966497] [PubMed: 27524885] [CrossRef]
- 156.
- Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376(15):1419-1429. doi:10.1056/NEJMoa1610187 [PMC free article: PMC5592722] [PubMed: 28402773] [CrossRef]
- 157.
- RISE Consortium, RISE Consortium Investigators. Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on β-cell function: comparison of responses in youth and adults. Diabetes. 2019;68(8):1670-1680. doi:10.2337/db19-0299 [PMC free article: PMC6692818] [PubMed: 31178433] [CrossRef]
- 158.
- TODAY Study Group; Bjornstad P, Drews KL, et al. Long-term complications in youth-onset type 2 diabetes. N Engl J Med. 2021;385(5):416-426. doi:10.1056/NEJMoa2100165 [PMC free article: PMC8697255] [PubMed: 34320286] [CrossRef]
- 159.
- RISE Consortium. Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care. 2018;41(8):1717-1725. doi:10.2337/dc18-0787 [PMC free article: PMC6054504] [PubMed: 29941500] [CrossRef]
- 160.
- Laffel LM, Danne T, Klingensmith GJ, et al. Efficacy and safety of the SGLT2 inhibitor empagliflozin versus placebo and the DPP-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (DINAMO): a multicentre, randomised, double-blind, parallel group, phase 3 trial. Lancet Diabetes Endocrinol. 2023;11(3):169-181. doi:10.1016/S2213-8587(22)00387-4 [PMC free article: PMC10851109] [PubMed: 36738751] [CrossRef]
- 161.
- Corcoran C, Jacobs TF. Metformin. StatPearls [Internet]. eBook. StatPearls Publishing; 2022. https://www
.ncbi.nlm .nih.gov/books/NBK518983/ - 162.
- Tamborlane WV, Barrientos-Pérez M, Fainberg U, et al. Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med. 2019;381(7):637-646. doi:10.1056/NEJMoa1903822 [PubMed: 31034184] [CrossRef]
- 163.
- Tamborlane WV, Bishai R, Geller D, et al. Once-weekly exenatide in youth with type 2 diabetes. Diabetes Care. 2022;45(8):1833-1840. doi:10.2337/dc21-2275 [PMC free article: PMC9346995] [PubMed: 35679098] [CrossRef]
- 164.
- Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202-210. doi:10.2337/ds16-0026 [PMC free article: PMC5556578] [PubMed: 28848315] [CrossRef]
- 165.
- Arslanian SA, Hannon T, Zeitler P, et al. Once-weekly dulaglutide for the treatment of youths with type 2 diabetes. N Engl J Med. 2022;387(5):433-443. doi:10.1056/NEJMoa2204601 [PubMed: 35658022] [CrossRef]
- 166.
- Tamborlane WV, Laffel LM, Weill J, et al. Randomized, double-blind, placebo-controlled dose-finding study of the dipeptidyl peptidase-4 inhibitor linagliptin in pediatric patients with type 2 diabetes. Pediatr Diabetes. 2018;19(4):640-648. doi:10.1111/pedi.12616 [PubMed: 29171139] [CrossRef]
- 167.
- Chadda KR, Cheng TS, Ong KK. GLP-1 agonists for obesity and type 2 diabetes in children: systematic review and meta-analysis. Obes Rev. 2021;22(6):e13177. doi:10.1111/obr.13177 [PubMed: 33354917] [CrossRef]
- 168.
- Shah AS, Zeitler PS, Wong J, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Type 2 Diabetes in Children and Adolescents. Pediatr Diabetes. 2022;23(7):872-902. doi:10.1111/pedi.13409 [PubMed: 36161685] [CrossRef]
- 169.
- Shah AS, Isom S, D’Agostino R, et al. Longitudinal changes in arterial stiffness and heart rate variability in youth-onset type 1 versus type 2 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care. 2022;45(7):1647-1656. doi:10.2337/dc21-2426 [PMC free article: PMC9274217] [PubMed: 35667385] [CrossRef]
- 170.
- Chan CL. Use of continuous glucose monitoring in youth-onset type 2 diabetes. Curr Diab Rep. 2017;17(9):66. doi:10.1007/s11892-017-0905-0 [PubMed: 28726154] [CrossRef]
- 171.
- Badaru A, Klingensmith GJ, Dabelea D, et al. Correlates of treatment patterns among youth with type 2 diabetes. Diabetes Care. 2014;37(1):64-72. doi:10.2337/dc13-1124 [PMC free article: PMC3867996] [PubMed: 24026554] [CrossRef]
- 172.
- Agarwal S, Raymond JK, Isom S, et al. Transfer from paediatric to adult care for young adults with type 2 diabetes: the SEARCH for Diabetes in Youth study. Diabet Med. 2018;35(4):504-512. doi:10.1111/dme.13589 [PMC free article: PMC6130201] [PubMed: 29377258] [CrossRef]
Appendix
Appendix Table A1.
Classes and Subgroups of Medications, Optum Clinformatics Data Mart and NHANES
Drs. Pihoker, Roberts, Zenno, and Le reported no conflicts of interest. Dr. Hirsch reported research funding from Dexcom and Insulet and consulting with Abbott, embecta, and Hagar.
Publication Details
Author Information and Affiliations
Authors
Irl B. Hirsch, MD, 1 Catherine Pihoker, MD,2 Alissa Roberts, MD,2 Anna Zenno, MD,1,2 and Phuc Le, PhD, MPH3,4.
1 Catherine Pihoker, MD,2 Alissa Roberts, MD,2 Anna Zenno, MD,1,2 and Phuc Le, PhD, MPH3,4.Affiliations
Department of Pediatrics
Seattle, WA
Primary Care Institute
Center for Value-Based Care Research
Cleveland, OH
Cleveland Clinic Lerner College of Medicine
Cleveland, OH
 Corresponding author.
Corresponding author.Publication History
Initial Posting: September 18, 2024.
Copyright
Diabetes in America is in the public domain of the United States. You may use the work without restriction in the United States.
Publisher
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Bethesda (MD)
NLM Citation
Hirsch IB, Pihoker C, Roberts A, et al. Medication Use and Self-Care Practices in Persons With Diabetes. 2024 Sep 18. In: Lawrence JM, Casagrande SS, Herman WH, et al., editors. Diabetes in America [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); 2023-.