Open Access: This content is Open Access under the Creative Commons license CC-BY-NC-ND.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Mattick J, Amaral P. RNA, the Epicenter of Genetic Information: A new understanding of molecular biology. Abingdon (UK): CRC Press; 2022 Sep 20. doi: 10.1201/9781003109242-18

RNA, the Epicenter of Genetic Information: A new understanding of molecular biology.
Show detailsIt seems that the nature of genetic information in complex organisms has been misunderstood since the inception of molecular biology, primarily because of the assumption that most genetic information is transacted by proteins. Other assumptions made during the formative years of genetics also appear to be incorrect, notably that mutations are random, and that epigenetic memory of experience is not inherited. Genomes contain biological software encompassing codes for components, self-assembly, development, cell differentiation and reproduction, supplemented by information in parental cells and epigenetic memories. Not only has the data evolved, but also the data structures, implementation systems and search algorithms. Indeed, it is likely that evolution has learned how to learn, and that many primitive preconceptions will have to be reevaluated, with more surprises in store.
“In science, ‘perverse’ cracking of pots is sometimes necessary to design new experiments, and to imagine new ideas that could help one to forge ahead, beyond the actual jungle of dogmas and preconceived opinions.”
(Klaus Scherrer 1 )
The Misunderstanding of Molecular Biology
It seems that the nature of genetic information in complex organisms has been misunderstood since the inception of molecular biology, because of the assumption that most genetic information is transacted by proteins. This assumption holds largely true for prokaryotes and to a lesser extent for eukaryotic microorganisms, which mainly must organize a cell to obtain nutrients and reproduce, albeit itself no mean feat. However, developmentally complex organisms, especially motile animals, have had to evolve much more sophisticated mechanisms to orchestrate cell differentiation and their assembly into highly organized ensembles. 2 , 3
The foundational assumption that genes (only) encode proteins led to many subsidiary assumptions, primarily that the vast tracts of non-coding and repetitive sequences in the genomes of complex organisms are graveyards of evolutionary junk colonized by molecular parasites. This rationalization was not disturbed by contrary genetic and molecular evidence assembled by Barbara McClintock, Roy Britten and Eric Davidson, Ed Lewis and others whose intuitions were ignored. It persisted in handwaving about the power of combinatorial control of gene expression by transcription factors. It persisted in founder fallacies and validation creep, a notable one being that developmental ‘enhancers’ bring transcription factors bound at their promoters into contact with the promoters of protein-coding genes whose expression they control, rather than the now increasingly clear alternative that they produce regulatory RNAs that organize local transcription and splicing hubs. Indeed, understanding enhancers is key to understanding the programming of differentiation and development.
Contrary to the view that the genomes of humans and other complex organisms are full of non-functional evolutionary detritus, they are in fact replete with information and dynamic activity. a The human genome makes trillions of cell fate decisions during development with high specificity and near-perfect reproducibility. This precision is effected by epigenetic mechanisms directed by regulatory RNAs, whose versatility may be largely achieved by programmed (cell state-specific) alternative splicing to alter target sites and modular recruitment of different types of effector proteins, incorporated into decisional hierarchies networked by sequences coopted from and distributed by transposable elements.
The separation of signal from consequent action, exemplified by RNAi and CRISPR, and writ large by enhancers and other types of lncRNAs, is a highly, and likely the most, efficient and versatile means of gene regulation. The advent of these advanced RNA-based regulatory systems permitted the emergence of developmentally complex organisms, following which evolution experimented with more sophisticated designs to colonize new niches, leading to the extraordinary biodiversity that we see today, embellished by sexual (mate) selection as proposed by Darwin. 4 Most differences between species and individuals are embedded in variations in their regulatory architecture, the extent of which expands with developmental complexity, like increasingly elaborate building plans using a relatively generic set of component parts, albeit with occasional important innovations, such as the immunoglobulin domain, Arc RNA transfer and RNA editing proteins.
Meanwhile, behind the scenes, while all organisms benefit from information processing, animals were climbing the next mountain, cognition, by superimposing plasticity on hardwired genomic information (Figure 18.1), with selection strength dependent on mobility and boosted by dexterity. 5 , 6 It may be no accident, contrary to Steven Jay Gould’s proposition of contingent history, 7–9 that the most cognitively advanced vertebrates and invertebrates are the primates and cephalopods.
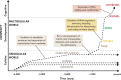
Figure 18.1
The evolution of complexity. (Image modified from Mattick.)
It is increasingly clear that, rather than a simple intermediate between gene and protein, RNA is the computational engine of the cell, development, cognition and evolution. 11 The challenge is not only to understand the principles of how RNA interacts with effector proteins in the formation and function of dedicated cellular domains to orchestrate cell division or differentiate decisions, but to decipher the code itself. This will be a bit like climbing inside a computer to try to work out how it can produce and project three-dimensional images, except that the human genome does so while building itself and its internal computer the brain, with far more precision and complexity, from an amazingly compact information suite that is roughly equivalent in size to that which can be held on a 1 Gb thumb drive.
The Evolution of Evolvability
The other long-standing assumptions have been that mutations are random, and that experience cannot be communicated to modulate the phenotype of subsequent generations, asserted in the formative years of evolutionary biology and molecular genetics based on preconception rather than evidence. Both assumptions are clearly incorrect, with non-random mutation and epigenetic inheritance now well documented in both plants and animals, 12–18 and evidence of a relationship. 19
This raises the question of whether there is interplay between genetic and epigenetic inheritance to accelerate evolutionary processes. It is obvious that evolution cannot have proceeded by random search alone – the number of variables is too great. This problem was recognized generically two decades ago by Rodney Downey and Michael Fellows, who pointed out that in large complex systems random searches become computationally intractable (‘NP-hard’) because of the exponential increase in the possibilities. 20 , 21 This problem must also apply to evolution if it operates, as described by Dennett, as a grinding algorithm of generate and test, 22 and becomes increasingly acute in organisms, notably birds and mammals, that have long generation times and small numbers of progeny. b
Downey and Fellows’s proposed solution to the problem is to define the most productive subspace and optimal tactics to decrease the complexity of the search and increase the chances of productive outcomes, termed ‘Parameterized Complexity’. 20 , 21 Logically, in the case of evolution, any (initially random) event that enhanced the evolvability of the lineage concerned must have been subject to second-order selection on the basis of its strategic advantage. By extension, any lineage that stumbled into such strategic advantage would come to dominate the evolutionary landscape and, by definition, be part of the toolkit of most, if not all, extant lineages in the biosphere.
The evidence to support this logic is fragmentary, and the topic of the evolution of evolvability has been subject to considerable speculation and debate. 26 Evolutionary computer science has shown that random mutation, recombination and selection are not universally effective in improving complex systems and that for adaptation to occur, these systems must acquire evolvability. 27 , 28 Moreover, the modeling suggests that simple genotype–phenotype mapping is suboptimal, whereas the use of indirect developmental representations allow the reuse of code (modularity), and scaling up of the complexity of artificially evolved phenotypes, for example, in robotics, artificial life and morphogenetic engineering. 29 Indeed one important enabler of biological evolution is modularity, 28 , 30–32 itself an evolved characteristic, 33 classically and graphically exemplified by simple homeotic mutations that convert insects from having two wings to four. 34
Different organisms have tuned their innate mutation frequencies to optimize the trade-off between survival and evolvability, 14 , 35 , 36 but this is only a blunderbuss approach. However, mutation frequency and transposon distribution vary across the genome and over time, 15 , 37 as judged by indices of neutral evolution, although these indices are unreliable and the extant distribution is difficult to disentangle from selection and differential repair and recombination. 16 , 38–42 Nonetheless, such variation occurs in the extensive non-coding regions of the genomes of complex organisms, evidence of selection at one level or another.
Rapid evolution has been documented in many species. It is, for example, observed in the human lineage, 43 correlating with bursts of Alu element invasion, reflecting the huge positive selection value of cognitive advancement in intra- and inter-species competition. 6 , 43
The interplay between genetic and epigenetic inheritance changes the dynamics of natural selection, as argued by Eva Jablonka and others. 44–50 It also changes the interplay between genes and environment, and the dichotomy of ‘Nature versus Nurture’ assessments, with profound evolutionary and social implications. Epigenome-associated mutation bias reduces the occurrence of deleterious mutations in essential genes in Arabidopsis 18 and recent evidence indicates that mutation sites in sperm are non-random, associated with adaptation. 51 There is also evidence that epigenetic changes can increase drug resistance in cancer cells until a genetic solution is found. 52
If epigenetic information can provide transgenerational phenotypic advantage, it is not a long stretch to suggest that evolution may have found ways to convert this information into hardwired genetic changes to speed adaptive change. In fact, it would be foolish to reject the possibility out of hand. The infrastructure – RNA-mediated epigenetic inheritance and RNA-templated DNA repair – is in place. Has evolution learned how to learn? 5
Genomes contain biological software encompassing codes for components, self-assembly, differentiation and reproduction, supplemented by information in parental cells and epigenetic memories. Not only has the data evolved, but also the data structures, implementation systems and search algorithms. We have some way to go to understand the complexity and beauty of genetic programming, but the best places to start are to accept that RNA plays a major role in the evolution and mechanics of developmental control and cognitive processes, and to keep an open and receptive mind, especially when we are once again surprised.
A new scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents eventually die, and a new generation grows up that is familiar with it.
(Max Planck 53 )
Further Reading
- Blount Z.D., Lenski R.E. and Losos J.B. (2018) Contingency and determinism in evolution: Replaying life’s tape. Science 362: eaam5979. [PubMed: 30409860]
- Dennett D. (1995) Darwin’s Dangerous Idea: Evolution and the Meanings of Life (Simon Schuster, New York).
- Erwin D.H. (2015) Novelty and innovation in the history of life. Current Biology 25: R930–940. [PubMed: 26439356]
- Jablonka E. (2017) The evolutionary implications of epigenetic inheritance. The Royal Society Interface Focus 7: 20160135. [PMC free article: PMC5566804] [PubMed: 28839916]
- Jablonka E. and Lamb M. (1994) Epigenetic Inheritance and Evolution—The Lamarckian Dimension (Oxford University Press, New York).
- Mattick J.S. (2007) A new paradigm for developmental biology. Journal of Experimental Biology 210: 1526–1547. [PubMed: 17449818]
- Mattick J.S. (2009) Deconstructing the dogma: A new view of the evolution and genetic programming of complex organisms. Annals of the New York Academy of Science 1178: 29–46. [PubMed: 19845626]
- Mattick J.S. (2009) Has evolution learnt how to learn? EMBO Reports 10: 665. [PMC free article: PMC2727432] [PubMed: 19568258]
- Mattick J.S. (2011) The central role of RNA in human development and cognition. FEBS Letters 585: 1600–1616. [PubMed: 21557942]
- Payne J.L. and Wagner A. (2019) The causes of evolvability and their evolution. Nature Reviews Genetics 20: 24–38. [PubMed: 30385867]
- Skinner M.K. (2015) Environmental epigenetics and a unified theory of the molecular aspects of evolution: A neo-Lamarckian concept that facilitates neo-Darwinian evolution. Genome Biology and Evolution 7: 1296–1302. [PMC free article: PMC4453068] [PubMed: 25917417]
Footnotes
- a
This is not to say there every sequence is functional. There will, of course, be recent duplications and transpositions that have not yet been subject to evolutionary selection.
- b
The numbers of variation options in such organisms per generation must be miniscule without compensatory mechanisms, such as the enigmatic double round of piRNA expression in sperm development, posited to allow controlled transposon mobilization and subsequent siRNA-mediated transcriptional proofing (which most sperm fail), to generate viable options for evolutionary selection in small populations with long generation times. 23 , 24 There are high primary rates of retrotransposition in mammals. 25
- Beyond the Jungle of Dogmas - RNA, the Epicenter of Genetic InformationBeyond the Jungle of Dogmas - RNA, the Epicenter of Genetic Information
Your browsing activity is empty.
Activity recording is turned off.
See more...