NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda (MD): National Heart, Lung, and Blood Institute (US); 2007 Aug.

Key Points
KEYPOINTS: MANAGING EXACERBATIONS OF ASTHMA
- ▪ Early treatment of asthma exacerbations is the best strategy for management. Important elements of early treatment at the patient's home include (EPR—2 1997):
- — Patient education, including a written asthma action plan to guide patient self-management of exacerbations at home, especially for patients who have moderate or severe persistent asthma and any patient who has a history of severe exacerbations (Evidence B). A peak-flow-based plan may be particularly useful for patients who have difficulty perceiving airflow obstruction and worsening asthma (Evidence D).
- — Recognition of early signs of worsening asthma and taking prompt action (Evidence A).
- — Appropriate intensification of therapy by increasing inhaled short-acting beta2-agonist (SABA) and, in some cases, adding a short course of oral systemic corticosteroids (Evidence A).
- — Removal or withdrawal of the environmental factor contributing to the exacerbation.
- — Prompt communication between patient and clinician about any serious deterioration in symptoms or peak flow, decreased responsiveness to SABAs, or decreased duration of effect.
- ▪ Management of asthma exacerbations requiring urgent medical care (e.g., in the urgent care setting or emergency department (ED)) includes:
- — Oxygen to relieve hypoxemia in moderate or severe exacerbations (EPR—2 1997).
- — SABA to relieve airflow obstruction, with addition of inhaled ipratropium bromide in severe exacerbations (Evidence A).
- — Systemic corticosteroids to decrease airway inflammation in moderate or severe exacerbations or for patients who fail to respond promptly and completely to a SABA (Evidence A).
- — Consideration of adjunct treatments, such as intravenous magnesium sulfate or heliox, in severe exacerbations unresponsive to the initial treatments listed above (Evidence B).
- — Monitoring response to therapy with serial measurements of lung function (Evidence B).
- — Preventing relapse of the exacerbation or recurrence of another exacerbation by providing: referral to followup asthma care within 1–4 weeks; an ED asthma discharge plan with instructions for medications prescribed at discharge and for increasing medications or seeking medical care if asthma worsens; review of inhaler techniques whenever possible; and consideration of initiating inhaled corticosteroids (ICSs) (Evidence B).
KEY DIFFERENCES FROM 1997 AND 2002 EXPERTPANEL REPORTS
- ▪ For the assessment of exacerbations, the current update (EPR—3: Full Report 2007):
- — Simplifies classification of severity of asthma exacerbations.
- — Reinstates, for use in the urgent or emergency care setting, the 1991 cut points of forced expiratory volume in 1 second (FEV1) or peak expiratory flow (PEF) to indicate the goal for discharge from the urgent care or emergency care setting (≥ 70 percent predicted FEV1 or PEF); patients for whom response to therapy is incomplete and who usually require continued treatment in the ED (40–69 percent predicted); and the exacerbation severity level where adjunct therapies may be considered (<40 percent predicted). These cut points differ from those used to determine long-term asthma control and treatments, thus underscoring the distinction between acute and chronic asthma management.
- — Acknowledges the limited value of pulmonary function measures in very severe exacerbations.
- ▪ For the treatment of exacerbations, the current update:
- — Adds levalbuterol as a SABA treatment for asthma exacerbations.
- — For home management of exacerbations, no longer recommends doubling the dose of ICSs.
- — For prehospital management (e.g., emergency transport), encourages standing orders for albuterol and—for prolonged transport—repeated treatments and protocols to allow consideration of ipratropium and oral corticosteroids.
- — For ED management, reduces dose and frequency of administration of oral corticosteroids in severe exacerbations, adds consideration of magnesium sulfate or heliox for severe exacerbations, and adds consideration of initiating an ICS upon discharge.
- — For hospital management, no longer recommends ipratropium bromide.
Introduction
In this section, recommendations are presented for the assessment and treatment of exacerbations in the home, ED, and hospital. See section 1, "Overall Methods Used To Develop This Report," for literature search strategy and tally of results for this EPR—3: Full Report 2007 section on "Managing Exacerbations of Asthma." Four Evidence Tables were prepared: 17, Increasing the Dose of Inhaled Corticosteroids; 18, IV Aminophylline; 19, Magnesium Sulfate; and 20, Heliox.
Asthma exacerbations are acute or subacute episodes of progressively worsening shortness of breath, cough, wheezing, and chest tightness—or some combination of these symptoms.
Exacerbations are characterized by decreases in expiratory airflow that can be documented and quantified by simple measurement of lung function (spirometry or PEF). These objective measures more reliably indicate the severity of an exacerbation than does the severity of symptoms. In general, milder exacerbations may be managed "at home" (i.e., outside the health care system), whereas more serious exacerbations may require an unscheduled ("urgent") office visit, an ED visit, or a hospital admission (see figure 5-1). The most severe exacerbations require admission to the intensive care unit (ICU) for optimal monitoring and treatment. Although assessment and treatment of young children pose unique challenges, the management of asthma exacerbations in older children and adults is fairly similar.

Figure 5-1
CLASSIFYING SEVERITY OF ASTHMA EXACERBATIONS IN THE URGENT OR EMERGENCY CARE SETTING
Individuals who have their asthma under control with ICSs will decrease the risk of exacerbations. Nonetheless, patients in good control can still be vulnerable to exacerbations, for example, when they have clinical respiratory infections (Reddel et al. 1999). Diurnal variability, a marker of poor control, may not change during an exacerbation; thus, clinicians may fail to detect important changes in lung function. The striking difference between PEF variations during exacerbations, as compared with during poor asthma control, suggests differences in beta2-adrenoceptor function between these conditions. The decrease in responsiveness to SABA during some severe exacerbations may help to explain the benefit of ipratropium bromide and other "alternative" approaches to bronchodilation.
General Considerations
Based on the scientific literature and the opinion of the Expert Panel, the Panel recommends that clinicians consider the following general principles and goals for managing asthma exacerbations: early treatment, special attention to patients who are at high risk of asthma-related death, and special attention to infants (EPR—2 1997).
- ▪ Early treatment is the best strategy for management of asthma exacerbations. Important elements of early treatment include:
- — A written asthma action plan (See "Component 2: Education for a Partnership in Asthma Care," figure 3–10) to guide patient self-management, especially for patients who have moderate or severe persistent asthma and any patient who has a history of severe exacerbations.
- — Recognition of early indicators of an exacerbation, including worsening PEF. Patients are instructed to recognize early signs and symptoms of worsening asthma and to use quick-relief medications if symptoms occur or if PEF drops below 80 percent predicted or personal best. If PEF is 50–79 percent, the patient should carefully monitor the response to quick-relief medication and, based on the response, consider contacting a clinician. If PEF is below 50 percent, immediate medical care is usually required (See sample written asthma action plans, figures 3–10a, b, and c.). In the urgent or emergency care setting, different parameters are used to classify the severity of the exacerbation and determine the clinical course; see figure 5-1. The Panel chose cut points of 40 percent and 70 percent of predicted (or personal best) because 40 percent denotes an exacerbation severity below which several adjunct therapies are effective, and 70 percent is a posttreatment goal for discharge from the ED or hospital.
- — Appropriate intensification of therapy, often including a short course of systemic corticosteroids.
- — Removal of or withdrawal from allergens or precipitating irritants in the environment that may be contributing to the exacerbation.
- — Prompt communication between patient and clinician about any serious deterioration in symptoms or peak flow, decreased responsiveness to SABA treatment, or decreased duration of effect.
- ▪ Patients who are at high risk for asthma-related death require special attention—particularly intensive education, monitoring, and care. Such patients should be counseled to seek medical care early during an exacerbation and instructed about the availability of ambulance services. Such patients include those who have identifiable risk factors (See figure 5-2a.).
- ▪ Infants require special attention, especially due to their greater risk for respiratory failure (See figure 5-2b.).

Figure 5-2a
RISK FACTORS FOR DEATH FROM ASTHMA Sources: Abramson et al. 2001; Greenberger et al. 1993; Hardie et al. 2002; Kallenbach et al. 1993; Kikuchi et al. 1994; O'Hollaren et al. 1991; Rodrigo and Rodrigo 1993; Strunk and Mrazek 1986; Suissa et al. 1994

Figure 5-2b
SPECIAL CONSIDERATIONS FOR INFANTS Source: EPR—2 1997.
Treatment Goals
The principal goals and Expert Panel recommendations for treating asthma exacerbations are:
- ▪ Correction of significant hypoxemia, in moderate or severe exacerbations, by administering supplemental oxygen. In rare instances, alveolar hypoventilation requires mechanically assisted ventilation (EPR—2 1997).
- ▪ Rapid reversal of airflow obstruction (Evidence A). This is best achieved by:
- — Repetitive or continuous administration of a SABA (Camargo et al. 2003b; Karpel et al. 1997; McFadden 2003; Travers et al. 2001)AND
- — Early in the course of treatment, administration of systemic corticosteroids to patients who have moderate or severe exacerbations or to patients who fail to respond promptly and completely to SABA treatment (McFadden 2003; Rachelefsky 2003; Rowe et al. 2004).
- ▪ Reduction of the likelihood of relapse of the exacerbation or future recurrence of severe airflow obstruction by intensifying therapy (Evidence A). Often, a short course of systemic corticosteroids is useful (Rachelefsky 2003; Rowe et al. 2004).
- ▪ Achieving these goals requires careful assessment and monitoring (Evidence B). The ability to predict care requirements, including the need for hospitalization, is based on repeated clinical assessments. In adults, repeated measurements of lung function are often helpful. The specific measurements chosen for monitoring will depend on the age of the patient and the available resources.
- — Children. In children, no single assessment tool appears to be the best for assessing the severity of the exacerbation or for monitoring response to treatment and predicting hospital admission (Chey et al. 1999; Gorelick et al. 2004a,b; Keahey et al. 2002; Keogh et al. 2001; Smith et al. 2002; Sole et al. 1999; Wright et al. 1997).
- ♦ Serial measurements of lung function. Lung function measures using either FEV1 or PEF may be useful for many children 5 years of age or older. However, neither maneuver may be obtainable during an exacerbation. Gorelick and colleagues (2004a) found that only 65 percent of children 5–18 years of age could complete either measurement during an acute exacerbation, and for children less than 5 years old, the maneuvers were almost impossible.
- ♦ Pulse oximetry. An initial pulse oximetry in infants and young children might be useful for assessing exacerbation severity but not for predicting the need for hospital admission (Keahey et al. 2002; Kelly et al. 2004; Keogh et al. 2001; Sole et al. 1999; Wright et al. 1997). However, a repeat pulse oximetry of <92–94 percent (sea level) at 1 hour was a better predictor of need for hospitalization, and it may be useful to move those infants and children into the hospital and out of the ED at that time (Kelly et al. 2004; Sole et al. 1999; Wright et al. 1997).
- ♦ Signs and symptoms scores. Several severity assessment scores have been developed and tested in children to help predict the need for hospitalization early in the course of ED treatment (Gorelick et al. 2004b; Keogh et al. 2001; Rodrigo and Rodrigo 1998b; Smith et al. 2002). None is 100 percent predictive, but the assessments may help to determine who should be transferred from the ED to the hospital after an initial 1- to 2-hour period of treatment, leaving the ED resources for those who are more likely to be able to go home after extended ED treatment and observation. These assessment scores combine physician- or nurse-observed signs and symptoms—such as shortness of breath, chest tightness, ability to speak in sentences or phrases, emotional impact, and alertness—plus respiratory rate, use of intercostals muscles, timing and volume of wheezes as well as pulse oximetry and, if available and feasible, FEV1 or PEF. One score (Gorelick et al. 2004b) has added a parental assessment of asthma control over the past several months and history of previous exacerbations requiring ED or hospital management. Others add the continuing need for hourly SABA 4 or more hours after the administration of oral systemic corticosteroids.A recent study suggests that most children who require hospitalization can be identified by a repeat assessment 1 hour after initial treatment (Kelly et al. 2004). After 1 hour, those children who continue to meet the criteria for a severe exacerbation have >86 percent chance of requiring hospitalization; those who meet the criteria for moderate exacerbation at 1 hour have an 84 percent chance of requiring hospitalization; and those whose assessment has remained the same or dropped to the mild level have only an 18 percent chance of requiring hospitalization. These severity assessment studies highlight the importance of regular, multifaceted assessments and close observation of children and adolescents who present to the office or ED with acute asthma exacerbations (See figures 5-1 and 5-3.).
- — Adults
- ♦ Serial measurements of lung function. FEV1 or PEF appear to be more useful in adults for categorizing the severity of the exacerbation and the response to treatment and in predicting the need for hospitalization. Repeated measurements of PEF or FEV1 in adults at 1 hour and beyond are useful as isolated assessments in determining who will require hospitalization and who is likely to have sufficient response to treatment to allow continued ED care. Indeed, repeated FEV1 or PEF measures at presentation to the ED and 1 hour after treatment were the strongest single predictor of hospitalization among adults who present to the ED with an asthma exacerbation (Karras et al. 2000; Kelly et al. 2004; McCarren et al. 2000; Rodrigo et al. 2004; Weber et al. 2002).When FEV1 is unavailable, PEF may be substituted. Although percentage of personal best PEF or FEV1 would be ideal for patient management, individuals may report erroneous values (Diner et al. 2001). Interpretation of percentage of predicted values is complicated by differences between formulas in the literature (Radeos and Camargo 2004). Regardless of the calculation chosen, for severe exacerbations with obvious airway compromise and even cyanosis, the immediate testing of FEV1 or PEF provides little additional information, and the maneuver can be very uncomfortable for acutely ill patients (Kelly et al. 2004).
- ♦ Pulse oximetry is indicated for patients who are in severe distress, have FEV1 or PEF <40 percent of predicted, or are unable to perform lung function measures.
- ♦ Signs and symptoms scores. As in children, some multifaceted prediction models have been tested and shown to improve slightly on the accuracy of the FEV1 or PEF alone (Chey et al. 1999; Kelly et al. 2002b and 2004; McFadden 2003; Weber et al. 2002). Kelly and colleagues (2004) used multiple signs and symptoms to determine the level of the severity of the exacerbation at 1 hour after the first ED treatment as well as the duration of symptoms (either <6 hours or ≥ 6 hours) before the patient's arrival at the ED (Kelly et al. 2002b) and found the additional measures improved the prediction rate by 5–10 percent. (See paragraph above related to the same model used in children.) For EDs that have limited resources, the presence of drowsiness in a patient is a useful predictor of impending respiratory failure and reason to consider immediate transfer of the patient to a facility equipped to deal with ventilatory support (Cham et al. 2002). As in the case with children, the ability to predict future care requirements is based on repeated clinical assessments, and, in adults, repeated measures of FEV1 or PEF (See figure 5-3.).

Figure 5-3
FORMAL EVALUATION OF ASTHMA EXACERBATION SEVERITY IN THE URGENT OR EMERGENCY CARE SETTING Notes:
Home Management of Asthma Exacerbations
Beginning treatment at home avoids treatment delays, prevents exacerbations from becoming severe, and also adds to patients' sense of control over their asthma. The degree of care provided in the home depends on the patients' (or parents') abilities and experience and on the availability of emergency care. General guidelines for managing exacerbations at home are presented in figure 5-4.
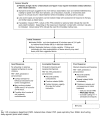
Figure 5-4
MANAGEMENT OF ASTHMA EXACERBATIONS: HOME TREATMENT
The Expert Panel recommends preparing patients for home management of asthma exacerbations by taking the following actions (Also see "Component 1: Measures of Asthma Assessment and Monitoring," and "Component 2: Education for a Partnership in Asthma Care.").
- ▪ Teach all patients how to monitor signs and symptoms so they can recognize early signs of deterioration and take appropriate action (Evidence A) (See "Component 2: Education for a Partnership in Asthma Care."), particularly since many fatal asthma exacerbations occur out of hospital (Krishnan et al. 2006). Patients should be taught how to adjust their medications early in an exacerbation (Kelly et al. 2002b) and when to call for further help or seek medical care. Patients should seek medical help earlier if the exacerbation is severe; treatment does not give rapid, sustained improvement; or there is further deterioration.
- ▪ Consider teaching how to monitor lung function, by using PEF to facilitate early and accurate assessment of exacerbations and response to treatment, to patients and parents of children who have moderate or severe persistent asthma or a history of severe exacerbations (Evidence B) and patients who are poor perceivers of airflow obstruction (Evidence D). Signs and symptoms imperfectly mirror airflow obstruction; therefore, other tools may be required, especially in the group of people who are "poor perceivers" and have failed to recognize previous exacerbations or symptom deteriorations early (Hardie et al. 2002; Kikuchi et al. 1994). Exacerbations recognized and treated within 6 hours of onset may be less likely to result in hospitalizations (Kelly et al. 2002b). When using PEF expressed only as a percent of personal best, the impact of any irreversible airflow obstruction must be considered. For example, in a person whose personal best is only 160 L/min, a drop to 60 percent of personal best represents life-threatening airflow obstruction.
- ▪ Provide to all patients a written asthma action plan that includes daily management and recognizing and handling worsening asthma, including self-adjustment of medications in response to acute symptoms or changes in PEF measures in the event of an exacerbation. A written asthma action plan is particularly recommended for patients who have moderate or severe persistent asthma and any patient who has a history of severe exacerbations or poorly controlled asthma (Evidence B). A peak-flow-based plan may be particularly useful for patients who have difficulty perceiving airflow obstruction and worsening asthma (Evidence D). See component 2—Education for a Partnership in Asthma Care, figure 3–10 for a sample plan. Children should also receive a plan appropriate to the school setting (See "Component 2: Education for a Partnership in Asthma Care," figure 3–16.). The plan should direct the patient to adjust medications in response to particular signs, symptoms, and peak flow measurements and should state when to seek medical help. Review the plan with the patient and family. The clinician should tailor the plan to the needs of individual patients. Patients who are at risk for asthma death (See figure 5-2a.) require especially close monitoring.
- ▪ Advise patients who have moderate or severe persistent asthma or a history of severe exacerbations to have the medication (e.g., corticosteroid tablets or liquid) and equipment (e.g., peak flow meter, compressor-driven nebulizer for young children) for treating exacerbations at home (Evidence A).
The Expert Panel recommends the following pharmacologic therapy for home management of exacerbations:
- ▪ Increase the frequency of SABA treatment (Evidence A).
- ▪ Initiate oral systemic corticosteroid treatment under certain circumstances (Evidence A). Short courses or "bursts" of oral corticosteroids reduce the duration and may prevent hospitalizations and relapse following an acute exacerbation (McFadden 2003; Rachelefsky 2003; Rowe et al. 2004). The Expert Panel recommends that, unless working from a defined action plan, individuals contact their health care provider before instituting a course of oral systemic corticosteroids (Evidence D).
- ▪ Doubling the ICS dose is not sufficient (Evidence B) (See Evidence Table 17, Increasing the Dose of Inhaled Corticosteroids.). Doubling the dose of an ICS in those patients already receiving ICS therapy has not been effective at reducing the severity or preventing progression of exacerbations (FitzGerald et al. 2004; Garrett et al. 1998; Harrison et al. 2004; Rice-McDonald et al. 2005). However, higher doses of an ICS may be effective in the ED management of acute asthma exacerbations. For example, preliminary evidence indicates that quadrupling the dose of an ICS for 7 days, starting at the first appearance of worsening symptoms, may prevent exacerbations requiring oral systemic corticosteroids (Foresi et al. 2000). For patients who experience substantial adverse effects with oral systemic corticosteroids (e.g., mood changes, worsening diabetes), high-dose ICS may be an effective alternative for mild to moderate exacerbations.
- ▪ Continue more intensive treatment for several days (EPR—2 1997). Recovery from an exacerbation varies, with symptom relief in 1–2 days for moderate exacerbations but in 3 or more days for severe exacerbations (See figure 5-1.). For many persons, the improvement is quite gradual. Even when symptoms have resolved, evidence of inflammation in the airways may continue for up to 2–3 weeks (McFadden 1975). In managing an exacerbation at home, patients' greater use of SABA should be continued until symptoms and PEF are stable. That said, patients should seek medical care rather than rely on bronchodilator therapy in excessive doses or for prolonged periods (e.g., >12 puffs/day for more than 24 hours).
The Expert Panel does not recommend the following home management techniques, because no studies have demonstrated effectiveness, and it is the opinion of the Panel that these techniques may delay patients from obtaining necessary care (EPR—2 1997).
- ▪ Drinking large volumes of liquids or breathing warm, moist air (e.g., the mist from a hot shower).
- ▪ Using over-the-counter products such as antihistamines or cold remedies. Over-the-counter bronchodilators may provide transient bronchodilation, but their use should not delay seeking medical care.
The Expert Panel also notes that although pursed-lip and other forms of controlled breathing may help to maintain calm during respiratory distress, these methods do not bring about improvement in lung function.
Prehospital Management of Asthma Exacerbations
The Expert Panel recommends that emergency medical services (EMS) providers administer supplemental oxygen and SABA to patients who have signs or symptoms of an asthma exacerbation (Evidence A).
Prehospital administration of SABA reduces airflow obstruction and relieves symptoms (Fergusson et al. 1995; Markenson et al. 2004; Richmond et al. 2005). Ideally, all EMS providers would receive a standing order to allow them to provide albuterol to their patients who have asthma exacerbations. Such an order would be consistent with their legally authorized scope of practice and local medical direction (Camargo 2006). In such settings, EMS providers should have available a nebulizer and/or an inhaler plus spacer/holding chamber for SABA administration (see figure 5-5 for dosages). If these are not available, subcutaneous epinephrine or terbutaline should be given for severe exacerbations (See figure 5-5.) (Sly et al. 1977; Smith et al. 1977).

Figure 5-5
DOSAGES OF DRUGS FOR ASTHMA EXACERBATIONS Notes:
When initiating bronchodilatory use, EMS personnel should not delay transport of the patient to the appropriate medical facility. The treatment, however, may be repeated while transporting the patient. Prolonged transport times (e.g., in a rural setting or during transport on congested urban streets) may necessitate multiple inhaled SABA treatments before arrival at the medical facility (Crago et al. 1998). Patients should receive a maximum of three inhaled SABA treatments in the first hour, and then one per hour thereafter (consistent with practice in the ED setting; see figures 5-5 and 5-6). After each treatment, EMS personnel should reassess and record the patient's vital signs and lung sounds.

Figure 5-6
MANAGEMENT OF ASTHMA EXACERBATIONS: EMERGENCY DEPARTMENT AND HOSPITAL-BASED CARE
Ambulance services should develop prehospital protocols for the treatment of acute asthma in children and adults (Markenson et al. 2004; Stead and Whiteside 1999). With medical oversight, these protocols can allow for more frequent administration of several established acute asthma treatments, such as ipratropium bromide and oral systemic corticosteroids (Knapp and Wood 2003). The latter medication is particularly important during prolonged transport times. All prehospital providers should receive training in how to respond to the clinical signs and symptoms of severe airway obstruction and imminent respiratory failure (Camargo 2006).
Emergency Department and Hospital Management of Asthma Exacerbations
Severe exacerbations of asthma are potentially life threatening. Care must be prompt. Effective initial therapies (i.e., SABA and the means of giving it by aerosol and a source of supplemental oxygen) should be available in a physician's office. Serious exacerbations, however, require close observation for deterioration, frequent treatment, and repetitive measurement of lung function. Therefore, most severe exacerbations of asthma require prompt transfer to an ED for more complete therapy (McFadden 2003; Rowe et al. 2001). Despite appropriate therapy, approximately 10–25 percent of ED patients who have acute asthma will require hospitalization (Pollack et al. 2002; Weber et al. 2002). In the hospital, multidisciplinary (e.g., nursing and respiratory care) clinical pathways for asthma appear to be effective in reducing hospital length-of-stay and inpatient costs, but they have less clear impact on clinical outcomes (Banasiak and Meadows-Oliver 2004). An overview of the treatment strategies in EDs and hospitals is presented in figure 5-6 and detailed below.
ASSESSMENT
The Expert Panel recommends the following activities to assess exacerbations:
- ▪ All clinicians treating patients who have asthma should be prepared to treat an asthma exacerbation, be familiar with the symptoms and signs of severe and life-threatening exacerbations (figures 5-1, 5-2a, and 5-3), and have procedures for facilitating immediate patient transfer to an emergency care facility (EPR—2 1997).
- ▪ Initial assessment should include a brief history, brief physical examination, and, for most patients, objective measures of lung function. Initial laboratory studies may be helpful, but they are not required for most patients, and they should not delay initiation of asthma treatment (EPR—2 1997).
- ▪ In the ED, all patients presenting with a reported asthma exacerbation must be evaluated and triaged immediately, based on at least vital signs and an overall physical assessment (e.g., ability to breathe well enough to talk). Treatment should begin immediately following recognition of a moderate, severe, or life-threatening exacerbation by assessment of symptoms, signs, or, when possible, lung function (EPR—2 1997).
- ▪ While treatment is given, obtain a brief, focused history and physical examination pertinent to the exacerbation (See figure 5-3.). Take a more detailed history and complete physical examination and perform laboratory studies only after initial therapy has been completed (Evidence D).
- ▪ The objectives of functional assessment (the frequency and number of measurements) will depend on the severity of the exacerbation and the response to treatment (See figure 5-6.) are to:
- — Obtain objective lung function measurements.
- ♦ FEV1 or PEF values provide important information about the level of airflow obstruction both initially and in response to treatment. Because low PEF values cannot distinguish between poor effort, restrictive ventilatory disorders (e.g., neuromuscular weakness, pneumonia), and obstructive ventilatory disorders (e.g., asthma), FEV1 measurements are preferable if they are readily available (Evidence D).
- ♦ In the initial assessment of a life-threatening asthma exacerbation, FEV1 or PEF are not indicated (Evidence D).
- ♦ Very severe exacerbations may preclude performance of a maximal expiratory maneuver and, in such cases, the clinical presentation should suffice for clinical assessment and prompt initiation of therapy (Evidence D).
- ♦ In less severe exacerbations, in the office or ED, FEV1 or PEF should be obtained on arrival and 30–60 minutes after initial treatment (Evidence B).
- ♦ In the hospital, FEV1 or PEF should be measured on admission and 15–20 minutes after bronchodilator therapy during the acute phase and at least daily thereafter until discharge (Evidence C).
- ♦ Any FEV1 or PEF value <25 percent of predicted that improves by <10 percent after treatment or values that fluctuate widely are potential indications for ICU admission and close monitoring for respiratory failure (Evidence C).
- ♦ Flow-volume loops should be obtained to distinguish between upper and lower airway obstruction in patients who have atypical asthma symptoms (e.g., dysphonia) or findings on exam (e.g., stridor) or if response to therapy is inadequate (Evidence D).
- — Monitor oxygen saturation.
- ♦ Pulse oximetry is indicated for children unable to perform FEV1 or PEF or for any patient who is in severe distress or has an FEV1 or PEF <40 percent of predicted to assess the adequacy of arterial oxygen saturation (SaO2) (Connett and Lenney 1993; Geelhoed et al. 1994; Sole et al. 1999; Wright et al. 1997) (Evidence C).
- ♦ Serial pulse oximetry measurements can be useful to assess both the severity of the exacerbation and improvement with treatment (Evidence B). By contrast, a single pulse oximetry value on admission is of relatively little value for predicting hospital admission (Boychuk et al. 2006; Keahey et al. 2002; Wright et al. 1997).
- ▪ Objectives of the brief history are to determine (EPR—2 1997):
- — Time of onset and any potential causes of current exacerbation.
- — Severity of symptoms, especially compared with previous exacerbations, and response to any treatment given before admission to ED.
- — All current medications and time of last dose, especially of asthma medications.
- — Estimate of number of previous unscheduled office visits, ED visits, and hospitalizations for asthma, particularly within the past year.
- — Any prior episodes of respiratory insufficiency due to asthma (loss of consciousness or intubation and mechanical ventilation).
- — Other potentially complicating illness, especially other pulmonary or cardiac disease or diseases that may be aggravated by systemic corticosteroid therapy (such as diabetes, peptic ulcer, hypertension, and psychosis).
- ▪ Objectives of the initial brief physical examination are to (EPR—2 1997):
- — Assess the severity of the exacerbation, as indicated by the findings listed in figure 5-3.
- — Assess overall patient status, including level of alertness, fluid status, and presence of cyanosis, respiratory distress, and wheezing. Wheezing can be an unreliable indicator of obstruction; in rare cases, extremely severe obstruction may be accompanied by a "silent chest" (Shim and Williams 1980).
- — Identify possible complications (e.g., pneumonia, pneumothorax, or pneumomediastinum); although rare, these will influence management of the asthma exacerbation.
- — Rule out upper airway obstruction. Both intrathoracic and extrathoracic central airway obstruction can cause severe dyspnea and may be diagnosed as asthma.
- ♦ Causes include upper airway foreign bodies, epiglottitis, organic diseases of the larynx, vocal cord dysfunction, and extrinsic and intrinsic tracheal narrowing (See "Component 1: Measures of Asthma Assessment and Monitoring.").
- ♦ Clues to the presence of alternative reasons for dyspnea include dysphonia, inspiratory stridor, monophonic wheezing loudest over the central airway, normal values for PO2, and unexpectedly complete resolution of airflow obstruction with intubation.
- ♦ When upper airway obstruction is suspected, further evaluation is indicated by flow-volume curves and by referral for laryngoscopy (See "Component 1: Measures of Asthma Assessment and Monitoring.").
- ▪ Laboratory studies. Most patients who have an asthma exacerbation do not require any initial laboratory studies. If laboratory studies are ordered, they must not delay initiation of asthma treatment (EPR—2 1997). The most important objective of laboratory studies is detection of actual or impending respiratory failure. Other objectives include detection of theophylline toxicity or conditions that complicate the treatment of asthma exacerbations (such as cardiovascular disease, pneumonia, or diabetes). For example:
- — Consider arterial blood gas (ABG) measurement for evaluating arterial carbon dioxide tension (PCO2) in patients who have suspected hypoventilation, severe distress, or FEV1 or PEF ≤25 percent of predicted after initial treatment. (Note: Respiratory drive is typically increased in asthma exacerbations, so a "normal" PCO2 of 40 mmHg indicates severe airflow obstruction and a heightened risk of respiratory failure.)
- — Venous levels of PCO 2 have been tested as a substitute for arterial measurements, and a venous PCO2 of >45 mmHg may serve as a screening test but cannot substitute for the ABG evaluation of respiratory function (Kelly et al. 2002a).
- — Complete blood count (CBC) is not required routinely but may be appropriate in patients who have fever or purulent sputum. Keep in mind that modest leukocytosis is common in asthma exacerbations and that corticosteroid treatment causes a further outpouring of polymorphonuclear leukocytes within 1–2 hours of administration.
- — Measure serum theophylline concentration in patients who have taken theophylline before presentation.
- — It may be prudent to measure serum electrolytes in patients who have been taking diuretics regularly and in patients who have coexistent cardiovascular disease, because frequent SABA administration can cause transient decreases in serum potassium, magnesium, and phosphate.
- — Chest radiography is not recommended for routine assessment but should be obtained for patients suspected of a complicating cardiopulmonary process, such as congestive heart failure, or another pulmonary process such as pneumothorax, pneumomediastinum, pneumonia, or lobar atelectasis.
- — Electrocardiograms are not required routinely, but a baseline electrocardiogram and continual monitoring of cardiac rhythm are appropriate in patients older than 50 years of age and in those who have coexistent heart disease or chronic obstructive pulmonary disease (COPD). The electrocardiogram may show a pattern of right ventricular strain that reverses promptly with treatment of airflow obstruction.
- ▪ Assessment considerations unique to children and infants are as follows:
- — It is often difficult for physicians and parents to determine the severity of the airway obstruction in infants and small children who have asthma. However, using a combination of the subjective and objective parameters in figure 5-3 permits a fairly accurate assessment to guide initial therapy. Many of these parameters have not been studied systematically, so they serve only as general guides.
- — The differences in the anatomy and physiology of the lungs of infants place them at greater risk for respiratory failure. These differences include greater peripheral airway resistance, fewer collateral channels of ventilation, further extension of airway smooth muscle into the peripheral airways, less elastic recoil, and mechanical disadvantage of the diaphragm. Viral infections, particularly respiratory syncytial virus (RSV), are the most common cause of acute wheezing illness in infants. The edematous inflammatory response in the airways leads to air trapping and hyperinflation, atelectasis, increased respiratory rate, and wheezing. This sequence of changes can rapidly progress to respiratory failure, and close monitoring is critical.
- — It is particularly important to monitor SaO2 by pulse oximetry in infants because their ventilation/perfusion characteristics lead them to become hypoxemic more readily than adults. SaO2 should be normal for altitude (>95 percent at sea level). Decreased SaO2 is often an early sign of severe airway obstruction, and an SaO2 <92 percent of room air 1 hour after initial treatment is a good predictor of the need for hospitalization in small infants (Connett and Lenney 1993; Geelhoed et al. 1994; Sole et al. 1999).
- — Capillary or ABG measurements should be performed on infants suspected of respiratory failure. PCO2 is the best measurement of ventilation in infants, as it is in adults. Children who have a normal PCO2 but are in obvious respiratory distress are at high risk for respiratory failure.
- — After initial treatment is begun, it is important to consider possible coexisting conditions, as is done in adults. In infants, considerations should include RSV infection, foreign body aspiration, history of bronchopulmonary dysplasia (prematurity) or cystic fibrosis.
TREATMENT
The Expert Panel recommends as initial treatments: oxygen for most patients, SABA for all patients; adding multiple doses of ipratropium bromide for ED patients who have severe exacerbations (but ipratropium bromide is not recommended during hospitalization); and systemic corticosteroids for most patients. For severe exacerbations unresponsive to the initial treatments, adjunct treatments (magnesium sulfate or heliox) merit consideration to decrease the likelihood of intubation. (See the following discussion for evidence levels.)
The Expert Panel does not recommend: methylxanthines, antibiotics (except as needed for comorbid conditions), aggressive hydration, chest physical therapy, mucolytics, or sedation. (For evidence levels, see the following discussion.)
In the ED and hospital, tailor the intensity of treatment and surveillance to the severity of the exacerbation. The primary therapies—the administration of oxygen, SABA, and systemic corticosteroids—are constant, but the dose and frequency with which they are given and the frequency with which the patient's response is assessed may vary. Thus, for patients presenting with a severe exacerbation, give SABA therapy at the higher dose plus ipratropium bromide (figure 5-5) either repeatedly (three treatments in the first hour) or continuously (by nebulization) (Evidence A). Give systemic corticosteroids immediately, and watch closely for signs of worsening airflow obstruction or fatigue. For patients who have mild exacerbations, give SABA therapy and assess the patient's response before deciding whether additional therapy is necessary. When SaO2 monitoring is not available, give supplemental oxygen to patients who have significant hypoxemia and to patients who have FEV1 or PEF <40 percent of predicted.
The Expert Panel recommends the following treatments:
- ▪ Oxygen is recommended for most patients (EPR—2 1997). Administer supplemental oxygen (by nasal cannulae or mask, whichever is best tolerated) to maintain an SaO2 >90 percent (>95 percent in pregnant women and in patients who have coexistent heart disease). Monitor SaO2 until a clear response to bronchodilator therapy has occurred.
- ▪ SABA treatment is recommended for all patients (Evidence A) (For recommended doses, see figure 5-5.).
- — The repetitive or continuous administration of SABAs is the most effective means of reversing airflow obstruction (Camargo et al. 2003b; Karpel et al. 1997; McFadden 2003; Travers et al. 2001).
- — In the ED, three treatments of SABA spaced every 20–30 minutes can be given safely as initial therapy. Thereafter, the frequency of administration varies according to the improvement in airflow obstruction and associated symptoms and the occurrence of side effects. Continuous administration of SABA may be more effective in more severely obstructed patients (Camargo et al. 2003b; Papo et al. 1993).
- — Because of the risk of cardiotoxicity, use only selective SABA (albuterol, levalbuterol, pirbuterol) in high doses.
- — In mild or moderate exacerbations, equivalent bronchodilation can be achieved either by high doses (4–12 puffs) of a SABA by MDI with a valved holding chamber (VHC) in infants, children, and adults under the supervision of trained personnel or by nebulizer therapy (Cates et al. 2003; Dolovich et al. 2005). However, nebulizer therapy may be preferred for patients who are unable to cooperate effectively in using an MDI because of their age, agitation, or severity of the exacerbation.
- — The onset of action for SABAs is less than 5 minutes; repetitive administration produces incremental bronchodilation. In about 60–70 percent of patients, response to the initial three doses in the ED will be sufficient to discharge them, and most patients will have a significant response after the first dose (Karpel et al. 1997; Rodrigo and Rodrigo 1998b; Strauss et al. 1997).
- — Duration of action of bronchodilation from SABAs in severe asthma exacerbations is not precisely known, but duration can be significantly shorter than that observed in stable asthma.
- — A recent meta-analysis of six trials suggests that the use of nebulized magnesium sulfate in combination with SABAs may result in further improvements in pulmonary function (Blitz et al. 2005), but further research is needed.
- ▪ Inhaled ipratropium bromide.
- — In the ED: recommended (Evidence A). Adding multiple high doses of ipratropium bromide (0.5 mg nebulizer solution or 8 puffs by MDI in adults; 0.25–0.5 mg nebulizer solution or 4–8 puffs by MDI in children) to a selective SABA produces additional bronchodilation, resulting in fewer hospital admissions, particularly in patients who have severe airflow obstruction (Plotnick and Ducharme 2000; Rodrigo and Castro-Rodriguez 2005).
- — In the hospital: not recommended (Evidence A). Two controlled clinical trials failed to detect a significant benefit from the addition of ipratropium to treatment after hospitalization for severe acute asthma (Craven et al. 2001; Goggin et al. 2001). Studies of hospitalized adults are not available.
- ▪ Systemic corticosteroids are recommended for most patients (For recommended doses, See figure 5-5.):
- — In the ED: Give systemic corticosteroids to patients who have moderate or severe exacerbations and patients who do not respond completely to initial SABA therapy (Evidence A). These medications speed the resolution of airflow obstruction and reduce the rate of relapse and may reduce hospitalizations (Edmonds et al. 2003; Rowe et al. 2001; Rowe et al. 2004).
- ♦ Oral administration of prednisone has been shown to have effects equivalent to those of intravenous methylprednisolone (Evidence A) (Harrison et al. 1986; Ratto et al. 1988) and, in the opinion of the Expert Panel, is usually preferred because it is less invasive.
- ♦ Give a 5- to 10-day course following ED discharge to prevent early relapse (EPR—2 1997).
- ♦ Intramuscular depot injections of corticosteroids may be considered as an alternative to oral corticosteroids for patients who are at high risk of nonadherence (Evidence D). Intramuscular depot injections may be as effective as oral corticosteroids for preventing relapse after discharge from the ED (Lahn et al. 2004; Rowe et al. 2001; Schuckman et al. 1998).
- ♦ Give supplemental doses of oral corticosteroids to patients who take them regularly, even if the exacerbation is mild (Evidence D).
- — In the hospital: Give systemic corticosteroids to patients who are admitted to the hospital (Evidence A), because oral systemic corticosteroids speed the resolution of asthma exacerbations (Manser et al. 2001; Smith et al. 2003).
- ▪ High doses of an ICS may be considered in the ED, although current evidence is insufficient to permit conclusions about using ICSs rather than oral systemic corticosteroids in the ED (Evidence B). (See Evidence Table 17, Increasing the Dose of Inhaled Corticosteroids.) Although simply doubling the dose of the ICS that a patient is taking for long-term therapy at the onset of an exacerbation does not appear to be effective (FitzGerald et al. 2004; Garrett et al. 1998; Harrison et al. 2004; Rice-McDonald et al. 2005), there is increasing evidence that multiple high doses of an ICS (6 mg flunisolide over 3 hours) (Rodrigo and Rodrigo 1998a) or 3 mg fluticasone/hour for 3 hours (Rodrigo 2005) are beneficial when initiated in adults early in the ED (See Evidence Table 17.). The data on ICS use in children are inconsistent (Rowe et al. 2004). This may be a result of the inconsistency of dosing. One trial reporting greater efficacy for oral corticosteroids used a single high dose of an ICS (2 mg fluticasone) (Schuh et al. 2000), whereas a trial giving multiple doses of budesonide (1.2 mg total) reported increased efficacy for the inhaled route (Singhi et al. 1999). The level of acute asthma severity also may explain apparent discrepancies found in the literature. Although the data are suggestive, a meta-analysis concluded that evidence was insufficient for firm conclusions (Edmonds et al. 2003). Further investigations with greater attention to dosing and acute asthma severity level are required.
- ▪ For severe exacerbations unresponsive to the initial treatments listed above, whether given before arrival at the acute care setting or in the ED, adjunct treatments may be considered to decrease the likelihood of intubation: intravenous magnesium or heliox may be useful (Evidence B). These therapies are discussed below, in the subsection on "Impending Respiratory Failure."
The following treatments are NOT recommended:
- ▪ Methylxanthines are not recommended (Evidence A). (See Evidence Table 18, IV Aminophylline.)
- — In the ED: Theophylline/aminophylline is not recommended because it appears to provide no additional benefit to optimal SABA therapy and increases the frequency of adverse effects (Parameswaran et al. 2000).
- — If patients are currently taking a theophylline-containing preparation, determine serum theophylline concentration to prevent theophylline toxicity.
- — In the hospital: Therapy with oral or intravenous methylxanthines does not improve lung function or other outcomes in hospitalized adults (Parameswaran et al. 2000). Most studies show no benefit, but increased toxicity, with theophylline in children who are hospitalized with severe asthma (Mitra et al. 2005). The meta-analysis, however, reported that those patients receiving intravenous aminophylline had a small (8–9 percent) but significant greater improvement in percent predicted FEV1. This difference was due to the weight of one study (Yung and South 1998), and this difference in lung function did not result in significant differences in length of stay, ICU admission or stay, or symptoms; however, significantly greater numbers of patients in the theophylline group had therapy discontinued due to adverse effects.
- ▪ Antibiotics are not generally recommended for the treatment of acute asthma exacerbations except as needed for comorbid conditions (Evidence B). Bacterial, Chlamydia, or Mycoplasma infections infrequently contribute to exacerbations of asthma; therefore, the use of antibiotics is generally reserved for patients who have fever and purulent sputum and for patients who have evidence of pneumonia (EPR—Update 2002). When the presence of bacterial sinusitis is strongly suspected, treat with antibiotics.
- ▪ Aggressive hydration is not recommended for older children and adults but may be indicated for some infants and young children (Evidence D). Intravenous or oral administration of large volumes of fluids does not play a role in the management of severe asthma exacerbations. Some infants and young children may become dehydrated, however, as a result of increased respiratory rate and decreased oral intake. In these patients, clinicians should make an assessment of fluid status (urine output, urine specific gravity, mucus membrane moisture, electrolytes) and provide appropriate corrections. The placement of intravenous lines is not without complication, and the emotional impact of this procedure may prove counterproductive. Oral routes of hydration are preferable except in very severe exacerbations with the possibility of endotracheal intubation.
- ▪ Chest physical therapy is not generally recommended (Evidence D). For most exacerbations, chest physiotherapy is not beneficial and is unnecessarily stressful for the breathless asthma patient. Because mucus plugging is a major contributing cause of fatal asthma (Kuyper et al. 2003), further studies are needed on the role of improved airway clearance in near-fatal exacerbations.
- ▪ Mucolytics are not recommended (Evidence C). Avoid mucolytic agents (e.g., acetylcysteine, potassium iodide) because they may worsen cough or airflow obstruction.
- ▪ Sedation is not generally recommended (Evidence D). Anxiolytic and hypnotic drugs are contraindicated in severely ill asthma patients because of their respiratory depressant effect. In asthmatic patients who have severe emotional impact, and possible comorbid anxiety disorder, therapy should stay focused on the asthma exacerbation; the benefit of short-acting sedatives is not known.
REPEAT ASSESSMENT
The Expert Panel recommends that repeat assessment of patients who have severe exacerbations be made after the initial dose of a SABA and that repeat assessment of all patients be made after three doses of a SABA (60–90 minutes after initiating treatment) (Evidence A).
The response to initial treatment in the ED is a better predictor of the need for hospitalization than is the severity of an exacerbation on presentation (Cham et al. 2002; Chey et al. 1999; Gorelick et al. 2004b; Karras et al. 2000; Kelly et al. 2002b and 2004; McCarren et al. 2000; Rodrigo and Rodrigo 1993, 1998c; Smith et al. 2002). The elements to be evaluated include the patient's subjective response, physical findings, FEV1 or PEF, and measurement of pulse oximetry or ABG (if the patient meets the criteria described in the earlier discussion of laboratory studies).
HOSPITALIZATION
The Expert Panel recommends that the decision to hospitalize a patient be based on duration and severity of symptoms, severity of airflow obstruction, response to ED treatment (See earlier section on monitoring in "Treatment Goals."), course and severity of prior exacerbations, medication use at the time of the exacerbation, access to medical care and medications, adequacy of support and home conditions, and presence of psychiatric illness (Evidence C) (Pollack et al. 2002; Weber et al. 2002.).
In general, the principles of care in the hospital and recommendation for treatment resemble those for care in the ED and involve both treatment (with oxygen, aerosolized SABA, and systemic corticosteroids and, perhaps, adjunct therapies) and frequent assessment, including clinical assessment of respiratory distress and fatigue as well as objective measurement of airflow (PEF or FEV 1 ) and oxygen saturation (EPR—2 1997).
IMPENDING RESPIRATORY FAILURE
The Expert Panel recommends that intubation not be delayed once it is deemed necessary; exactly when to intubate is based on clinical judgment (Evidence D). Most patients respond well to therapy. However, a small minority will show signs of worsening ventilation, whether from worsening airflow obstruction, worsening respiratory muscle fatigue, or a combination of the two. Signs of impending respiratory failure include inability to speak, altered mental status, intercostal retraction (Cham et al. 2002), worsening fatigue, and a PCO2 of ≥42 mmHg. Because respiratory failure can progress rapidly and can be difficult to reverse, early recognition and treatment are critically important.
The Expert Panel recommends that adjunct treatments such as magnesium sulfate or heliox may be considered to avoid intubation, but intubation should not be delayed once it is deemed necessary (Evidence B). Because intubation of a severely ill asthma patient is difficult and associated with complications, additional treatments are sometimes attempted.
- ▪ Intravenous magnesium sulfate. Consider intravenous magnesium sulfate in patients who have life-threatening exacerbations and in those whose exacerbations remain in the severe category after 1 hour of intensive conventional therapy (Evidence B). (See Evidence Table 19, Magnesium Sulfate.) Meta-analyses of studies of both children and adults (Cheuk et al. 2005; Rowe et al. 2000) show that intravenous magnesium sulfate (2 grams in adults and 25–75 mg/kg up to 2 grams in children) added to conventional therapy reduces hospitalization rates in ED patients who present with severe asthma exacerbations (PEF <40 percent). However, not all individual studies have found positive results (Boonyavorakul et al. 2000; Porter et al. 2001; Scarfone et al. 2000). The treatment has no apparent value in patients who have exacerbations of lesser severity, and one study (Silverman et al. 2002) found that intravenous magnesium sulfate improved pulmonary function only in patients whose initial FEV1 was <25 percent predicted, and the treatment did not improve hospital admission rates.
- ▪ Heliox. Consider heliox-driven albuterol nebulization for patients who have life-threatening exacerbations and for those patients whose exacerbations remain in the severe category after 1 hour of intensive conventional therapy (Evidence B). (See Evidence Table 20, Heliox.)
- Because of helium's low density, a mixture of helium and oxygen (heliox) could improve gas exchange in patients who have airway obstruction (Gupta and Cheifetz 2005). However, a meta-analysis of six studies (four in adults, two in pediatric patients) performed between 1996 and 2002 did not find a statistically significant improvement in pulmonary function or other measured outcomes in patients receiving heliox compared to oxygen or air (Ho et al. 2003). Likewise, a more recent study did not demonstrate a statistically significant benefit in children who had moderately severe asthma and received standard initial therapy followed by continuous albuterol nebulization with heliox compared to air/oxygen (Rivera et al. 2006). In contrast, another recent study (Kim et al. 2005) did report a significant improvement in pulmonary index and a trend toward reduced hospitalizations in children who had moderate-to-severe exacerbations and received heliox-driven albuterol nebulization compared to children who received oxygen-driven nebulization. Other investigators recently described two randomized controlled trials (RCTs) of adults that demonstrated more rapid and greater improvements in peak flow and dyspnea scores in patients who presented with severe exacerbations and received initial treatment with heliox versus oxygen-driven albuterol therapy (Lee et al. 2005). The discrepancy in findings may result from small sample sizes. More importantly, however, some studies have neglected to account for the different effect of heliox versus oxygen (or room air) on respirable mass (Hess et al. 1999). For example, failure to increase the gas flow rate for those on heliox greatly complicates interpretation (and synthesis) of the literature.
- ▪ Other adjunct therapies to avoid intubation include intravenous beta 2 -agonists, intravenous leukotriene receptor antagonists (LTRAs), and noninvasive ventilation; however, insufficient data are available to make recommendations regarding these possible adjunct therapies (Evidence D).
- — Intravenous beta2-agonists remain a largely unproven treatment. Current evidence does not suggest an improved benefit from intravenous beta2-agonists compared to aerosol administration (Travers et al. 2001), but data are sparse (Browne et al. 1997) on the benefit of adding an intravenous beta2-agonist to high-dose nebulized therapy. Nevertheless, the Expert Panel does not recommend use of intravenous isoproterenol in the treatment of asthma because of the danger of myocardial toxicity (Evidence B) (Maguire et al. 1991).
- — Intravenous LTRAs could provide another pathway to rapid bronchodilation during impending respiratory failure. A randomized trial of intravenous montelukast in moderate and severe exacerbations demonstrated significant improvement in pulmonary function within 10 minutes of administration (Camargo et al. 2003a). The oral formulation LTRA would not be expected to provide benefit for at least 90 minutes (Dockhorn et al. 2000).
- — Noninvasive ventilation is another experimental approach for treatment of respiratory failure due to severe asthma exacerbation, but data are very limited (Ram et al. 2005).
- — Review of other experimental adjunct therapies is beyond the scope of this report.
The Expert Panel recommends the following actions regarding intubation:
- ▪ Patients who present with apnea or coma should be intubated immediately (EPR—2 1997). There are no other absolute indications for endotracheal intubation, but persistent or increasing hypercapnia, exhaustion, and depression of mental status strongly suggest the need for ventilatory support (Evidence D).
- ▪ Intubate semielectively, before the crisis of respiratory arrest, because intubation is difficult in patients who have asthma (EPR—2 1997).
- ▪ Intubation should be performed by a physician who has extensive experience in intubation and airway management (EPR 2 1997).
- — Because intubation should not be delayed once it is deemed necessary, it is often performed in the ED or inpatient ward, and the patient is subsequently transferred to an ICU appropriate to the patient's age.
- — Children who are intubated for asthma should be admitted to a pediatric ICU or transferred to a facility that has such a unit.
- — Even without intubation, patients who have severe exacerbations and are slow to respond to therapy may benefit from admission to an ICU, where they can be monitored closely and intubated if it is indicated.
- — Despite theoretical benefits from using ketamine as a premedication for intubation, clinical trials in nonintubated patients who have severe exacerbations have not shown clinical benefit (Allen and Macias 2005; Howton et al. 1996). Studies of intubated patients are not available.
- — Although many issues require consideration at the time of intubation, clinicians should pay close attention to maintaining or replacing intravascular volume, because hypotension commonly accompanies the initiation of positive pressure ventilation.
- ▪ "Permissive hypercapnia" or "controlled hypoventilation" is the recommended ventilator strategy (Evidence C). Permissive hypercapnia provides adequate oxygenation and ventilation while minimizing high airway pressures and barotrauma (Darioli and Perret 1984; Menitove and Goldring 1983; Tuxen 1994). It involves administration of as high a fraction of inspired oxygen as is necessary to maintain adequate arterial oxygenation, acceptance of hypercapnia, and treatment of respiratory acidosis with intravenous sodium bicarbonate. Adjustments are made to the tidal volume, ventilator rate, and inspiration-to-expiration ratio to minimize airway pressures. Consultation with or comanagement by physicians who have expertise in ventilator management is appropriate, because mechanical ventilation of patients who have severe refractory asthma is complicated and fraught with risk. Continuation of a SABA in ventilated patients is recommended, although no RCTs provide evidence for or against this practice (Dhand and Tobin 1997; Jones et al. 2001). This ventilator strategy is not uniformly successful in critically ill asthma patients, and additional therapies are being evaluated. Their review is beyond the scope of this report.
PATIENT DISCHARGE
The Expert Panel recommends that clinicians, before patients' discharge from the ED or hospital, provide patients with necessary medications and education on how to use them, a referral for a followup appointment, and instruction in an ED asthma discharge plan for recognizing and managing relapse of the exacerbation or recurrence of airflow obstruction (Evidence B).
The Expert Panel recommends the following actions for discharging patients from the ED:
- ▪ Release of the patient from the ED depends on the patient's response to treatment (EPR 2 1997).
- — In general, discharge is appropriate if FEV1 or PEF has returned to ≥70 percent of predicted or personal best and symptoms are minimal or absent. Patients who have an incomplete response to therapy (FEV1 or PEF 50–69 percent of predicted or personal best) and with mild symptoms should be assessed individually for their suitability for discharge home, with consideration given to factors listed in figure 5-2a (Evidence C).
- — The Expert Panel's opinion is that patients who have a rapid response should be observed for 30–60 minutes after the most recent dose of bronchodilator to ensure their stability of response before discharge to home.
- — Extended treatment and observation in a holding area, clinical decision unit, or overnight unit to determine the need for hospitalization may be appropriate, provided there is sufficient monitoring and nursing care (McCarren et al. 2000).
- ▪ Prescribe sufficient medications for the patient to continue treatment after discharge.
- — Patients given systemic corticosteroids should continue oral systemic corticosteroids for 3–10 days (Evidence A). The need for further corticosteroid therapy should be assessed at a followup visit. For corticosteroid courses of less than 1 week, there is no need to taper the dose. For 10-day courses, there remains no need to taper if patients are concurrently taking ICSs (O'Driscoll et al. 1993).
- — Consider initiating an ICS at discharge, in addition to oral systemic corticosteroids (Evidence B). A retrospective review of a large patient database found a significant reduction in the risk of subsequent ED visits among patients using ICS therapy after ED discharge (Sin and Man 2002). A clinical RCT comparing ED patients discharged with and without ICSs demonstrated that ICSs added to oral systemic corticosteroids halved patients' risk of relapse events (Rowe et al. 1999). A Cochrane review (Edmonds et al. 2000) noted that two other relapse trials did not report similar benefit, but the review found that the combined estimate of the three available trials had borderline statistical significance (odds ratio 0.68; 95 percent CI 0.46 to 1.02). The Expert Panel concludes that initiating ICS therapy (e.g., providing a 1–2 month supply) at discharge from ED should be considered, given the potential for ICSs is to reduce subsequent ED visits, the strong evidence that long-term-control ICS therapy reduces exacerbations in patients who have persistent asthma, and the opinion of the Expert Panel that the initiation (and continuation) of ICS therapy at ED discharge can be an important effort to bridge the gap between emergency and primary care for asthma. Patients already taking ICS therapy should continue it following discharge.
- ▪ Emphasize the need for continual, regular care in an outpatient setting, and refer the patient for a followup asthma care appointment (either primary care provider (PCP) or asthma specialist) within 1–4 weeks (Evidence B). If appropriate, consider referral to an asthma self-management education program (Evidence B). A visit to the ED is often an indication of inadequate long-term management of asthma or inadequate plans for handling exacerbations. Having fewer general practice contacts in the previous year has been independently associated with an increased risk of fatal asthma (Sturdy et al. 2005), and an observational study found that having followup appointments within 30 days of an asthma-related ED visit was associated with a reduced 90-day readmission rate (Sin et al. 2002). Likewise, referral of patients in the ED to an asthma specialist for consultation was associated with a reduced rate of subsequent ED visits (Zeiger et al. 1991). These results contrast, however, with two recent randomized trials that found that facilitated referral of ED patients to the PCP did not alter long-term asthma outcomes (Baren et al. 2006; Smith et al. 2004). Although the evidence from RCTs regarding optimal referral practice is limited (e.g., PCP or asthma specialist), the ED and hospital staff should notify the patient's health care professional (or provide a referral to one if the patient does not name a source of asthma care) and encourage the patient to contact his/her health care provider (e.g., by telephone) for asthma-related problems during the first 3–5 days after ED or hospital discharge. The ED and hospital staff should instruct the patient to seek a followup medical appointment within 1–4 weeks. Whenever possible, the ED should schedule such an appointment before the patient is discharged, because this action will increase the likelihood that the patient actually receives an appointment and attends the followup (Baren et al. 2006; Zorc et al. 2003). At the followup appointment, the health care provider should try to ascertain the cause of the exacerbation and institute appropriate, specific, preventative therapy if possible. The followup visit should also include a detailed review of the patient's medications, inhaler and peak flow meter technique, and development of a comprehensive written asthma action plan that will help prevent subsequent exacerbations and urgent or emergency care visits (See section 3, "Component 2: Education for a Partnership in Asthma Care," figures 3–10a, b, and c; 3–11; and 3–14.). If appropriate, consider referring the patient to an asthma self-management education program.
- ▪ Review discharge medications with the patient and provide patient education on correct use of an inhaler (Evidence B) (See section 3, "Component 2: Education for a Partnership in Asthma Care," figures 3–12 and 3–14.).
- ▪ Give the patient an ED asthma discharge plan with instruction for medications prescribed at discharge and for increasing medications or seeking medical care if asthma should worsen (Evidence B). Although evidence from RCTs is limited, for many patients, a thoughtful, asthma-oriented ED discharge plan will suffice. If local staff and resources permit, however, the provision of a more detailed plan may be appropriate, especially for patients who had severe exacerbations or who do not have regular asthma care. See figure 5-7 for a sample ED asthma discharge plan and "Component 2: Education for a Partnership in Asthma Care."
- ▪ Consider issuing a peak flow meter and giving appropriate education on how to measure and record PEF to patients who have difficulty perceiving airflow obstruction or symptoms of worsening asthma (Evidence D). Studies document that some patients are unable to perceive signs of deterioration that would indicate a need to increase medication (Hardie et al. 2002; Kikuchi et al. 1994). These "poor perceivers" may particularly benefit from action plans based on peak flow monitoring, because this tool may prevent delays in treating exacerbations. Although clinical trials have not yet evaluated issuing peak flow meters at discharge from ED, it is the opinion of the Expert Panel that this approach warrants consideration. See "Component 1: Measures of Asthma Assessment and Monitoring" and "Component 2: Education for a Partnership in Asthma Care" for discussions of the advantages and disadvantages of peak flow monitoring.

Figure 5-7
EMERGENCY DEPARTMENT – ASTHMA DISCHARGE PLAN Source: Camargo CA Jr, Emond SD, Boulet L, Gibson PG, Kolbe J, Wagner CW, Brenner BE. Emergency Department Asthma Action Plan. Developed at "Asthma Education in the Adult Emergency Department: A Multidisciplinary (more...)
The Expert Panel recommends the following actions for discharging patients from the hospital:
- ▪ Prior to discharge, adjust the patient's medication to an outpatient regimen (EPR—2 1997). During the first 24 hours after this medication adjustment, observe the patient for possible deterioration.
- ▪ Discharge medications should include a SABA and sufficient oral systemic corticosteroids to complete the course of therapy (Evidence A) and instructions to continue long-term control therapy until the followup appointment (Evidence B). Consider initiating ICS therapy for patients who did not use an ICS prior to the hospital admission (Evidence B). If the decision is made to start the patient on an ICS, the ICS should be started before the course of oral corticosteroids is completed, because their onset of action is gradual (Kraan et al. 1988). Starting the ICS therapy before discharge gives the patient additional time to learn and demonstrate appropriate technique.
- ▪ Provide patient education:
- — Review patient understanding of the causes of asthma exacerbations, the purposes and correct uses of treatment (including inhaler technique), and the actions to be taken for worsening symptoms or peak flow measures (Evidence B) (See "Component 2: Education for a Partnership in Asthma Care."). An exacerbation severe enough to require hospitalization may reflect a failure of the patient's self-management, particularly in patients who have low levels of health literacy (Paasche-Orlow et al. 2005). Some studies report that 35 percent of adult patients presenting to the ED are current smokers (Silverman et al. 2003). It would be appropriate to query patients hospitalized for asthma about their smoking status and encourage smoking cessation along with their asthma discharge plan. Hospitalized patients may be particularly receptive to information and advice about their illness (See "Component 2: Education for a Partnership in Asthma Care.").
- — Educate patients about their discharge medications and the importance of taking medications as prescribed and attending their followup visit (Evidence B). Low levels of adherence to asthma medications are common, even in patients recently hospitalized for severe asthma exacerbations (Krishnan et al. 2004).
- — Referral to an asthma specialist should be considered for patients who have a history of life-threatening exacerbations or multiple hospitalizations (Evidence B) (Harish et al. 2001; Mahr and Evans 1993; Mayo et al. 1990; Sperber et al. 1995).
- — Consider issuing a peak flow meter and giving appropriate education on peak flow monitoring to patients who are ≥5 years of age (and parents) who have a history of severe exacerbations or who have moderate or severe persistent asthma (Evidence B) and those who poorly perceive airflow obstruction or worsening asthma (Evidence D).
- ▪ Review or develop a written plan for managing either relapse of the exacerbation of recurrent symptoms or exacerbations (Evidence B). The plan should describe the signs, symptoms, and/or peak flow values that should prompt increases in self-medication, contact with a health care provider, or return for emergency care. The plan given at discharge from the ED may be quite simple (e.g., instructions for discharge medications and returning for care if asthma worsens; see figure 5-7b). The preparation for discharge from the hospital should be more complete (See figure 5-8.). A detailed written asthma action plan for comprehensive long-term management and handling of exacerbations should be developed by the regular provider at a followup visit (See figure 3–10a, b, and c; "Component 2: Education for a Partnership in Asthma Care.").

Figure 5-7b
EMERGENCY DEPARTMENT – ASTHMA DISCHARGE PLAN : HOW TO USE YOUR METERED - DOSE INHALER

Figure 5-8
CHECK LIST FOR HOSPITAL DISCHARGE OF PATIENTS WHO HAVE ASTHMA
References
- Abramson MJ, Bailey MJ, Couper FJ, Driver JS, Drummer OH, Forbes AB, McNeil JJ, Haydn WE. Victorian Asthma Mortality Study Group. Are asthma medications and management related to deaths from asthma? Am J Respir Crit Care Med. 2001;163(1):12–8. [PubMed: 11208619]
- Allen JY, Macias CG. The efficacy of ketamine in pediatric emergency department patients who present with acute severe asthma. Ann Emerg Med. 2005;46(1):43–50. [PubMed: 15988425]
- Banasiak NC, Meadows-Oliver M. Inpatient asthma clinical pathways for the pediatric patient: an integrative review of the literature. Pediatr Nurs. 2004;30(6):447–50. [PubMed: 15704591]
- Baren JM, Boudreaux ED, Brenner BE, Cydulka RK, Rowe BH, Clark S, Camargo CA Jr. Randomized controlled trial of emergency department interventions to improve primary care follow-up for patients with acute asthma. Chest. 2006;129(2):257–65. [PubMed: 16478839]
- Blitz M, Blitz S, Hughes R, Diner B, Beasley R, Knopp J, Rowe BH. Aerosolized magnesium sulfate for acute asthma: a systematic review. Chest. 2005;128(1):337–44. [PubMed: 16002955]
- Boonyavorakul C, Thakkinstian A, Charoenpan P. Intravenous magnesium sulfate in acute severe asthma. Respirology. 2000;5(3):221–5. [PubMed: 11022983]
- Boychuk RB, Yamamoto LG, DeMesa CJ, Kiyabu KM. Correlation of initial emergency department pulse oximetry values in asthma severity classes (steps) with the risk of hospitalization. Am J Emerg Med. 2006;24(1):48–52. [PubMed: 16338509]
- Browne GJ, Penna AS, Phung X, Soo M. Randomised trial of intravenous salbutamol in early management of acute severe asthma in children. Lancet. 1997;349(9048):301–5. [PubMed: 9024371]
- Camargo CA Jr. A model protocol for emergency medical services management of asthma exacerbations. Prehosp Emerg Care. 2006;10(4):418–29. [PubMed: 16997769]
- Camargo CA Jr, Emond SD, Boulet L, Gibson PG, Kolbe J, Wagner CW, Brenner BE. Emergency Department Asthma Action Plan. Developed at "Asthma Education in the Adult Emergency Department: A Multidisciplinary Consensus Conference," New York Academy of Medicine, New York, NY; 2001 April 1–5. Boston, MA: Massachusetts General Hospital, 2001. 2 pp.
- Camargo CA Jr,, Smithline HA, Malice MP, Green SA, Reiss TF. A randomized controlled trial of intravenous montelukast in acute asthma. Am J Respir Crit Care Med. 2003a;167(4):528–33. [PubMed: 12456380]
- Camargo CA Jr, Spooner CH, Rowe BH. Continuous versus intermittent beta-agonists in the treatment of acute asthma. Cochrane Database Syst Rev. 2003b;(4):CD001115. [PMC free article: PMC8407022] [PubMed: 14583926]
- Cates CC, Bara A, Crilly JA, Rowe BH. Holding chambers versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2003;(3):CD000052. [PubMed: 12917881]
- Cham GW, Tan WP, Earnest A, Soh CH. Clinical predictors of acute respiratory acidosis during exacerbation of asthma and chronic obstructive pulmonary disease. Eur J Emerg Med. 2002;9(3):225–32. [PubMed: 12394618]
- Cheuk DK, Chau TC, Lee SL. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child. 2005;90(1):74–7. [PMC free article: PMC1720072] [PubMed: 15613519]
- Chey T, Jalaludin B, Hanson R, Leeder S. Validation of a predictive model for asthma admission in children: how accurate is it for predicting admissions? J Clin Epidemiol. 1999;52(12):1157–63. [PubMed: 10580778]
- Connett GJ, Lenney W. Use of pulse oximetry in the hospital management of acute asthma in childhood. Pediatr Pulmonol. 1993;15(6):345–9. [PubMed: 8337012]
- Crago S, Coors L, Lapidus JA, Sapien R, Murphy SJ. Prehospital treatment of acute asthma in a rural state. Ann Allergy Asthma Immunol. 1998;81(4):322–5. [PubMed: 9809495]
- Craven D, Kercsmar CM, Myers TR, O'Riordan MA, Golonka G, Moore S. Ipratropium bromide plus nebulized albuterol for the treatment of hospitalized children with acute asthma. J Pediatr. 2001;138(1):51–8. [PubMed: 11148512]
- Darioli R, Perret C. Mechanical controlled hypoventilation in status asthmaticus. Am Rev Respir Dis. 1984;129(3):385–37. [PubMed: 6703497]
- Dhand R, Tobin MJ. Inhaled bronchodilator therapy in mechanically ventilated patients. Am J Respir Crit Care Med. 1997;156(1):3–10. [PubMed: 9230718]
- Diner B, Brenner B, Camargo CA Jr. Inaccuracy of "personal best" peak expiratory flow rate reported by inner-city patients with acute asthma. J Asthma. 2001;38(2):127–32. [PubMed: 11321682]
- Dockhorn RJ, Baumgartner RA, Leff JA, Noonan M, Vandormael K, Stricker W, Weinland DE, Reiss TF. Comparison of the effects of intravenous and oral montelukast on airway function: a double blind, placebo controlled, three period, crossover study in asthmatic patients. Thorax. 2000;55(4):260–5. [PMC free article: PMC1745728] [PubMed: 10722763]
- Dolovich MB, Ahrens RC, Hess DR, Anderson P, Dhand R, Rau JL, Smaldone GC, Guyatt G. American College of Chest Physicians; American College of Asthma, Allergy, and Immunology. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–71. [PubMed: 15654001]
- Edmonds ML, Camargo CA Jr, Pollack CV Jr, Rowe BH. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database Syst Rev. 2003;(3):CD002308. [PubMed: 12917930]
- Edmonds ML, Camargo CA Jr, Saunders LD, Brenner BE, Rowe BH. Inhaled steroids in acute asthma following emergency department discharge. Cochrane Database Syst Rev. 2000;(3):CD002316. [PubMed: 10908556]
- EPR—2. Expert panel report 2: guidelines for the diagnosis and management of asthma (EPR—2 1997). NIH Publication No. 97-4051. Bethesda, MD: U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program, 1997.
- EPR—Update 2002. Expert panel report: guidelines for the diagnosis and management of asthma. Update on selected topics 2002 (EPR—Update 2002). NIH Publication No. 02-5074. Bethesda, MD: U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program, June 2003. Available at http://www
.nhlbi.nih .gov/guidelines/asthma/asthmafullrpt.pdf. - Fergusson RJ, Stewart CM, Wathen CG, Moffat R, Crompton GK. Effectiveness of nebulised salbutamol administered in ambulances to patients with severe acute asthma. Thorax. 1995;50(1):81–2. [PMC free article: PMC473719] [PubMed: 7886656]
- FitzGerald JM, Becker A, Sears MR, Mink S, Chung K, Lee J. Doubling the dose of budesonide versus maintenance treatment in asthma exacerbations. Thorax. 2004;59(7):550–6. [PMC free article: PMC1747069] [PubMed: 15223858]
- Foresi A, Morelli MC, Catena E. Low-dose budesonide with the addition of an increased dose during exacerbations is effective in long-term asthma control. On behalf of the Italian Study Group. Chest. 2000;117(2):440–6. [PubMed: 10669688]
- Garrett J, Williams S, Wong C, Holdaway D. Treatment of acute asthmatic exacerbations with an increased dose of inhaled steroid. Arch Dis Child. 1998;79(1):12–7. [PMC free article: PMC1717626] [PubMed: 9771245]
- Geelhoed GC, Landau LI, Le Souef PN. Evaluation of SaO2 as a predictor of outcome in 280 children presenting with acute asthma. Ann Emerg Med. 1994;23(6):1236–41. [PubMed: 8198296]
- Goggin N, Macarthur C, Parkin PC. Randomized trial of the addition of ipratropium bromide to albuterol and corticosteroid therapy in children hospitalized because of an acute asthma exacerbation. Arch Pediatr Adolesc Med. 2001;155(12):1329–34. [PubMed: 11732951]
- Gorelick MH, Stevens MW, Schultz T, Scribano PV. Difficulty in obtaining peak expiratory flow measurements in children with acute asthma. Pediatr Emerg Care. 2004a;20(1):22–6. [PubMed: 14716161]
- Gorelick MH, Stevens MW, Schultz TR, Scribano PV. Performance of a novel clinical score, the Pediatric Asthma Severity Score (PASS), in the evaluation of acute asthma. Acad Emerg Med. 2004b;11(1):10–8. [PubMed: 14709423]
- Greenberger PA, Miller TP, Lifschultz B. Circumstances surrounding deaths from asthma in Cook County (Chicago) Illinois. Allergy Proc. 1993;14(5):321–6. [PubMed: 8288113]
- Gupta VK, Cheifetz IM. Heliox administration in the pediatric intensive care unit: an evidence-based review. Pediatr Crit Care Med. 2005;6(2):204–11. [PubMed: 15730610]
- Hardie GE, Gold WM, Janson S, Carrieri-Kohlman V, Boushey HA. Understanding how asthmatics perceive symptom distress during a methacholine challenge. J Asthma. 2002;39(7):611–8. [PubMed: 12442950]
- Harish Z, Bregante AC, Morgan C, Fann CS, Callaghan CM, Witt MA, Levinson KA, Caspe WB. A comprehensive inner-city asthma program reduces hospital and emergency room utilization. Ann Allergy Asthma Immunol. 2001;86(2):185–9. [PubMed: 11258688]
- Harrison BD, Stokes TC, Hart GJ, Vaughan DA, Ali NJ, Robinson AA. Need for intravenous hydrocortisone in addition to oral prednisolone in patients admitted to hospital with severe asthma without ventilatory failure. Lancet. 1986;1(8474):181–4. [PubMed: 2868207]
- Harrison TW, Oborne J, Newton S, Tattersfield AE. Doubling the dose of inhaled corticosteroid to prevent asthma exacerbations: randomised controlled trial. Lancet. 2004;363(9405):271–5. [PubMed: 14751699]
- Hess DR, Acosta FL, Ritz RH, Kacmarek RM, Camargo CA Jr. The effect of heliox on nebulizer function using a beta-agonist bronchodilator. Chest. 1999;115(1):184–9. [PubMed: 9925082]
- Ho J, Bender BG, Gavin LA, O'Connor SL, Wamboldt MZ, Wamboldt FS. Relations among asthma knowledge, treatment adherence, and outcome. J Allergy Clin Immunol. 2003;111(3):498–502. [PubMed: 12642828]
- Howton JC, Rose J, Duffy S, Zoltanski T, Levitt MA. Randomized, double-blind, placebo-controlled trial of intravenous ketamine in acute asthma. Ann Emerg Med. 1996;27(2):170–5. [PubMed: 8629747]
- Jones A, Rowe B, Peters J, Camargo C, Hammarquist C, Rowe B. Inhaled beta-agonists for asthma in mechanically ventilated patients. Cochrane Database Syst Rev. 2001;(4):CD001493. [PubMed: 11687115]
- Kallenbach JM, Frankel AH, Lapinsky SE, Thornton AS, Blott JA, Smith C, Feldman C, Zwi S. Determinants of near fatality in acute severe asthma. Am J Med. 1993;95(3):265–72. [PubMed: 8368225]
- Karpel JP, Aldrich TK, Prezant DJ, Guguchev K, Gaitan-Salas A, Pathiparti R. Emergency treatment of acute asthma with albuterol metered-dose inhaler plus holding chamber: how often should treatments be administered? Chest. 1997;112(2):348–56. [PubMed: 9266868]
- Karras DJ, Sammon ME, Terregino CA, Lopez BL, Griswold SK, Arnold GK. Clinically meaningful changes in quantitative measures of asthma severity. Acad Emerg Med. 2000;7(4):327–34. [PubMed: 10805619]
- Keahey L, Bulloch B, Becker AB, Pollack CV Jr,, Clark S, Camargo CA Jr. Initial oxygen saturation as a predictor of admission in children presenting to the emergency department with acute asthma. Ann Emerg Med. 2002;40(3):300–7. [PubMed: 12192354]
- Kelly AM, Kerr D, Powell C. Is severity assessment after one hour of treatment better for predicting the need for admission in acute asthma? Respir Med. 2004;98(8):777–81. [PubMed: 15303644]
- Kelly AM, Kyle E, McAlpine R. Venous pCO(2) and pH can be used to screen for significant hypercarbia in emergency patients with acute respiratory disease. J Emerg Med. 2002a;22(1):15–9. [PubMed: 11809551]
- Kelly AM, Powell C, Kerr D. Patients with a longer duration of symptoms of acute asthma are more likely to require admission to hospital. Emerg Med (Fremantle ). 2002b;14(2):142–5. [PubMed: 12147110]
- Keogh KA, Macarthur C, Parkin PC, Stephens D, Arseneault R, Tennis O, Bacal L, Schuh S. Predictors of hospitalization in children with acute asthma. J Pediatr. 2001;139(2):273–7. [PubMed: 11487756]
- Kikuchi Y, Okabe S, Tamura G, Hida W, Homma M, Shirato K, Takishima T. Chemosensitivity and perception of dyspnea in patients with a history of near-fatal asthma. N Engl J Med. 1994;330(19):1329–34. [PubMed: 8152444]
- Kim IK, Phrampus E, Venkataraman S, Pitetti R, Saville A, Corcoran T, Gracely E, Funt N, Thompson A. Helium/oxygen-driven albuterol nebulization in the treatment of children with moderate to severe asthma exacerbations: a randomized, controlled trial. Pediatrics. 2005;116(5):1127–33. [PubMed: 16263999]
- Knapp B, Wood C. The prehospital administration of intravenous methylprednisolone lowers hospital admission rates for moderate to severe asthma. Prehosp Emerg Care. 2003;7(4):423–6. [PubMed: 14582090]
- Kraan J, Koeter GH, van der Mark TW, Boorsma M, Kukler J, Sluiter HJ, De Vries K. Dosage and time effects of inhaled budesonide on bronchial hyperreactivity. Am Rev Respir Dis. 1988;137(1):44–8. [PubMed: 3276257]
- Krishnan JA, Riekert KA, McCoy JV, Stewart DY, Schmidt S, Chanmugam A, Hill P, Rand CS. Corticosteroid use after hospital discharge among high-risk adults with asthma. Am J Respir Crit Care Med. 2004;170(12):1281–15. [PubMed: 15374842]
- Krishnan V, Diette GB, Rand CS, Bilderback AL, Merriman B, Hansel NN, Krishnan JA. Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med. 2006;174(6):633–8. [PMC free article: PMC2648055] [PubMed: 16778163]
- Kuyper LM, Pare PD, Hogg JC, Lambert RK, Ionescu D, Woods R, Bai TR. Characterization of airway plugging in fatal asthma. Am J Med. 2003;115(1):6–11. [PubMed: 12867228]
- Lahn M, Bijur P, Gallagher EJ. Randomized clinical trial of intramuscular vs oral methylprednisolone in the treatment of asthma exacerbations following discharge from an emergency department. Chest. 2004;126(2):362–8. [PubMed: 15302718]
- Lee DL, Hsu CW, Lee H, Chang HW, Huang YC. Beneficial effects of albuterol therapy driven by heliox versus by oxygen in severe asthma exacerbation. Acad Emerg Med. 2005;12(9):820–7. [PubMed: 16141015]
- Maguire JF, O'Rourke PP, Colan SD, Geha RS, Crone R. Cardiotoxicity during treatment of severe childhood asthma. Pediatrics. 1991;88(6):1180–6. [PubMed: 1956735]
- Mahr TA, Evans R III. Allergist influence on asthma care. Ann Allergy. 1993;71(2):115–20. [PubMed: 8346862]
- Manser R, Reid D, Abramson M. Corticosteroids for acute severe asthma in hospitalised patients. Cochrane Database Syst Rev. 2001;(1):CD001740. [PubMed: 11279726]
- Markenson D, Foltin G, Tunik M, Cooper A, Treiber M, Caravaglia K. Albuterol sulfate administration by EMT-basics: results of a demonstration project. Prehosp Emerg Care. 2004;8(1):34–40. [PubMed: 14691785]
- Mayo PH, Richman J, Harris HW. Results of a program to reduce admissions for adult asthma. Ann Intern Med. 1990;112(11):864–71. [PubMed: 2344111]
- McCarren M, Zalenski RJ, McDermott M, Kaur K. Predicting recovery from acute asthma in an emergency diagnostic and treatment unit. Acad Emerg Med. 2000;7(1):28–35. [PubMed: 10894239]
- McFadden ER Jr. The chronicity of acute attacks of asthma—mechanical and therapeutic implications. J Allergy Clin Immunol. 1975;56(1):18–26. [PubMed: 1138017]
- McFadden ER Jr. Acute severe asthma. Am J Respir Crit Care Med. 2003;168(7):740–759. [PubMed: 14522812]
- Menitove SM, Goldring RM. Combined ventilator and bicarbonate strategy in the management of status asthmaticus. Am J Med. 1983;74(5):898–901. [PubMed: 6837612]
- Mitra A, Bassler D, Goodman K, Lasserson TJ, Ducharme FM. Intravenous aminophylline for acute severe asthma in children over two years receiving inhaled bronchodilators. Cochrane Database Syst Rev. 2005;(2):CD001276. Review. [PMC free article: PMC7027703] [PubMed: 15846615]
- O'Driscoll BR, Kalra S, Wilson M, Pickering CA, Carroll KB, Woodcock AA. Double-blind trial of steroid tapering in acute asthma. Lancet. 1993;341(8841):324–7. [PubMed: 8094111]
- O'Hollaren MT, Yunginger JW, Offord KP, Somers MJ, O'Connell EJ, Ballard DJ, Sachs MI. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med. 1991;324(6):359–63. [PubMed: 1987459]
- Paasche-Orlow MK, Riekert KA, Bilderback A, Chanmugam A, Hill P, Rand CS, Brancati FL, Krishnan JA. Tailored education may reduce health literacy disparities in asthma self-management. Am J Respir Crit Care Med. 2005;172(8):980–6. Epub August 2005. [PMC free article: PMC2718412] [PubMed: 16081544]
- Papo MC, Frank J, Thompson AE. A prospective, randomized study of continuous versus intermittent nebulized albuterol for severe status asthmaticus in children. Crit Care Med. 1993;21(10):1479–86. [PubMed: 8403956]
- Parameswaran K, Belda J, Rowe BH. Addition of intravenous aminophylline to beta2-agonists in adults with acute asthma. Cochrane Database Syst Rev. 2000;(4):CD002742. [PubMed: 11034753]
- Plotnick LH, Ducharme FM. Combined inhaled anticholinergics and beta2-agonists for initial treatment of acute asthma in children. Cochrane Database Syst Rev. 2000;(4):CD000060. [PubMed: 11034671]
- Pollack CV Jr, Pollack ES, Baren JM, Smith SR, Woodruff PG, Clark S, Camargo CA Jr. Multicenter Airway Research Collaboration Investigators. A prospective multicenter study of patient factors associated with hospital admission from the emergency department among children with acute asthma. Arch Pediatr Adolesc Med. 2002;156(9):934–40. [PubMed: 12197803]
- Porter RS, Nester, Braitman LE, Geary U, Dalsey WC. Intravenous magnesium is ineffective in adult asthma, a randomized trial. Eur J Emerg Med. 2001;8(1):9–15. [PubMed: 11314826]
- Rachelefsky G. Treating exacerbations of asthma in children: the role of systemic corticosteroids. Pediatrics. 2003;112(2):382–97. [PubMed: 12897291]
- Radeos MS, Camargo CA Jr. Predicted peak expiratory flow: differences across formulae in the literature. Am J Emerg Med. 2004;22(7):516–21. [PubMed: 15666252]
- Ram FS, Wellington S, Rowe BH, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2005;(1):CD004360. [PubMed: 16034928]
- Ratto D, Alfaro C, Sipsey J, Glovsky MM, Sharma OP. Are intravenous corticosteroids required in status asthmaticus? JAMA. 1988;260(4):527–9. [PubMed: 3385910]
- Reddel H, Ware S, Marks G, Salome C, Jenkins C, Woolcock A. Differences between asthma exacerbations and poor asthma control. Lancet. 1999;353(9150):364–9. [PubMed: 9950442]
- Rice-McDonald G, Bowler S, Staines G, Mitchell C. Doubling daily inhaled corticosteroid dose is ineffective in mild to moderately severe attacks of asthma in adults. Intern Med J. 2005;35(12):693–8. [PubMed: 16313543]
- Richmond NJ, Silverman R, Kusick M, Matallana L, Winokur J. Out-of-hospital administration of albuterol for asthma by basic life support providers. Acad Emerg Med. 2005;12(5):396–403. [PubMed: 15860692]
- Ritz T, Steptoe A, Dewilde S, Costa M. Emotions and stress increase respiratory resistance in asthma. Psychosom Med. 2000;62(3):401–12. [PubMed: 10845354]
- Rivera ML, Kim TY, Stewart GM, Minasyan L, Brown L. Albuterol nebulized in heliox in the initial ED treatment of pediatric asthma: a blinded, randomized controlled trial. Am J Emerg Med. 2006;24(1):38–42. [PubMed: 16338507]
- Rodrigo C, Rodrigo G. Salbutamol treatment of acute severe asthma in the ED: MDI versus hand-held nebulizer. Am J Emerg Med. 1998a;16(7):637–42. [PubMed: 9827736]
- Rodrigo C, Rodrigo G. Therapeutic response patterns to high and cumulative doses of salbutamol in acute severe asthma. Chest. 1998b;113(3):593–8. [PubMed: 9515830]
- Rodrigo G, Rodrigo C. Assessment of the patient with acute asthma in the emergency department. A factor analytic study. Chest. 1993;104(5):1325–8. [PubMed: 8222781]
- Rodrigo G, Rodrigo C. Early prediction of poor response in acute asthma patients in the emergency department. Chest. 1998c;114(4):1016–21. [PubMed: 9792570]
- Rodrigo GJ. Comparison of inhaled fluticasone with intravenous hydrocortisone in the treatment of adult acute asthma. Am J Respir Crit Care Med. 2005;171(11):1231–6. Epub March 2005. [PubMed: 15764724]
- Rodrigo GJ, Castro-Rodriguez JA. Anticholinergics in the treatment of children and adults with acute asthma: a systematic review with meta-analysis. Thorax. 2005;60(9):740–6. Epub July 2005. Erratum in: Thorax 2006;61(3):274 and Thorax 2006;61(5)458. [PMC free article: PMC1747524] [PubMed: 16055613]
- Rodrigo GJ, Rodrigo C, Hall JB. Acute asthma in adults: a review. Chest. 2004;125(3):1081–102. [PubMed: 15006973]
- Rowe BH, Bota GW, Fabris L, Therrien SA, Milner RA, Jacono J. Inhaled budesonide in addition to oral corticosteroids to prevent asthma relapse following discharge from the emergency department: a randomized controlled trial. JAMA. 1999;281(22):2119–26. [PubMed: 10367823]
- Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA Jr. Intravenous magnesium sulfate treatment for acute asthma in the emergency department: a systematic review of the literature. Ann Emerg Med. 2000;36(3):181–90. [PubMed: 10969218]
- Rowe BH, Edmonds ML, Spooner CH, Camargo CA Jr. Evidence-based treatments for acute asthma. Respir Care. 2001;46(12):1380–1390. Discussion 1390–1. [PubMed: 11728298]
- Rowe BH, Edmonds ML, Spooner CH, Diner B, Camargo CA Jr. Corticosteroid therapy for acute asthma. Respir Med. 2004;98(4):275–84. [PubMed: 15072167]
- Scarfone RJ, Loiselle JM, Joffe MD, Mull CC, Stiller S, Thompson K, Gracely EJ. A randomized trial of magnesium in the emergency department treatment of children with asthma. Ann Emerg Med. 2000;36(6):572–8. [PubMed: 11097697]
- Schuckman H, DeJulius DP, Blanda M, Gerson LW, DeJulius AJ, Rajaratnam M. Comparison of intramuscular triamcinolone and oral prednisone in the outpatient treatment of acute asthma: a randomized controlled trial. Ann Emerg Med. 1998;31(3):333–8. [PubMed: 9506490]
- Schuh S, Reisman J, Alshehri M, Dupuis A, Corey M, Arseneault R, Alothman G, Tennis O, Canny G. A comparison of inhaled fluticasone and oral prednisone for children with severe acute asthma. N Engl J Med. 2000;343(10):689–94. [PubMed: 10974132]
- Shim CS, Williams MH Jr. Evaluation of the severity of asthma: patients versus physicians. Am J Med. 1980;68(1):11–3. [PubMed: 7350797]
- Silverman RA, Boudreaux ED, Woodruff PG, Clark S, Camargo CA Jr. Cigarette smoking among asthmatic adults presenting to 64 emergency departments. Chest. 2003;123(5):1472–9. [PubMed: 12740263]
- Silverman RA, Osborn H, Runge J, Gallagher EJ, Chiang W, Feldman J, Gaeta T, Freeman K, Levin B, Mancherje N. Acute Asthma/Magnesium Study Group. et al. IV magnesium sulfate in the treatment of acute severe asthma: a multicenter randomized controlled trial. Chest. 2002;122(2):489–97. [PubMed: 12171821]
- Sin DD, Bell NR, Svenson LW, Man SF. The impact of follow-up physician visits on emergency readmissions for patients with asthma and chronic obstructive pulmonary disease: a population-based study. Am J Med. 2002;112(2):120–5. [PubMed: 11835950]
- Sin DD, Man SF. Low-dose inhaled corticosteroid therapy and risk of emergency department visits for asthma. Arch Intern Med. 2002;162(14):1591–5. [PubMed: 12123402]
- Singhi S, Banerjee S, Nanjundaswamy H. Inhaled budesonide in acute asthma. J Paediatr Child Health. 1999;35(5):483–7. [PubMed: 10571764]
- Sly RM, Badiei B, Faciane J. Comparison of subcutaneous terbutaline with epinephrine in the treatment of asthma in children. J Allergy Clin Immunol. 1977;59(2):128–35. [PubMed: 319140]
- Smith M, Iqbal S, Elliott TM, Everard M, Rowe BH. Corticosteroids for hospitalised children with acute asthma. Cochrane Database Syst Rev. 2003;(1):CD002886. [PMC free article: PMC6999806] [PubMed: 12804441]
- Smith PR, Heurich AE, Leffler CT, Henis MM, Lyons HA. A comparative study of subcutaneously administered terbutaline and epinephrine in the treatment of acute bronchial asthma. Chest. 1977;71(2):129–34. [PubMed: 318965]
- Smith SR, Baty JD, Hodge D III. Validation of the pulmonary score: an asthma severity score for children. Acad Emerg Med. 2002;9(2):99–104. [PubMed: 11825832]
- Smith SR, Jaffe DM, Fisher EB Jr,, Trinkaus KM, Highstein G, Strunk RC. Improving follow-up for children with asthma after an acute Emergency Department visit. J Pediatr 2004;145(6):772–7. Erratum in: J Pediatr. 2005;146(3):413. [PubMed: 15580199]
- Sole D, Komatsu MK, Carvalho KV, Naspitz CK. Pulse oximetry in the evaluation of the severity of acute asthma and/or wheezing in children. J Asthma. 1999;36(4):327–33. [PubMed: 10386496]
- Sperber K, Ibrahim H, Hoffman B, Eisenmesser B, Hsu H, Corn B. Effectiveness of a specialized asthma clinic in reducing asthma morbidity in an inner-city minority population. J Asthma. 1995;32(5):335–43. [PubMed: 7559274]
- Stead L, Whiteside T. Evaluation of a new EMS asthma protocol in New York City: a preliminary report. Prehosp Emerg Care. 1999;3(4):338–42. [PubMed: 10534036]
- Strauss L, Hejal R, Galan G, Dixon L, McFadden ER Jr. Observations on the effects of aerosolized albuterol in acute asthma. Am J Respir Crit Care Med. 1997;155(2):454–458. [PubMed: 9032178]
- Strunk RC, Mrazek DA. Deaths from asthma in childhood: can they be predicted? N Engl Reg Allergy Proc. 1986;7(5):454–61. [PubMed: 3503205]
- Sturdy PM, Butland BK, Anderson HR, Ayres JG, Bland JM, Harrison BD, Peckitt C, Victor CR. National Asthma Campaign Mortality and Severe Morbidity Group. Deaths certified as asthma and use of medical services: a national case-control study. Thorax. 2005;60(11):909–15. Epub July 2005. [PMC free article: PMC1747248] [PubMed: 16055628]
- Suissa S, Ernst P, Boivin JF, Horwitz RI, Habbick B, Cockroft D, Blais L, McNutt M, Buist AS, Spitzer WO. A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am J Respir Crit Care Med. 1994;149(3 Pt 1):604–10. [PubMed: 8118625]
- Travers A, Jones AP, Kelly K, Barker SJ, Camargo CA, Rowe BH. Intravenous beta2-agonists for acute asthma in the emergency department. Cochrane Database Syst Rev. 2001;(2):CD002988. [PMC free article: PMC8406466] [PubMed: 11406055]
- Tuxen DV. Permissive hypercapnic ventilation. Am J Respir Crit Care Med. 1994;150(3):870–4. [PubMed: 8087364]
- von Leupoldt A, Dahme B. Emotions and airway resistance in asthma: study with whole body plethysmography. Psychophysiology. 2005;42(1):92–7. [PubMed: 15720584]
- Weber EJ, Silverman RA, Callaham ML, Pollack CV Jr,, Woodruff PG, Clark S, Camargo CA Jr. A prospective multicenter study of factors associated with hospital admission among adults with acute asthma. Am J Med. 2002;113(5):371–8. [PubMed: 12401531]
- Wright RO, Santucci KA, Jay GD, Steele DW. Evaluation of pre- and posttreatment pulse oximetry in acute childhood asthma. Acad Emerg Med. 1997;4(2):114–7. [PubMed: 9043537]
- Yung M, South M. Randomised controlled trial of aminophylline for severe acute asthma. Arch Dis Child. 1998;79(5):405–10. [PMC free article: PMC1717748] [PubMed: 10193252]
- Zeiger RS, Heller S, Mellon MH, Wald J, Falkoff R, Schatz M. Facilitated referral to asthma specialist reduces relapses in asthma emergency room visits. J Allergy Clin Immunol 1991;87(6):1160–8. Erratum in: J Allergy Clin Immunol. 1992;90(2):278. [PubMed: 2045618]
- Zorc JJ, Scarfone RJ, Li Y, Hong T, Harmelin M, Grunstein L, Andre JB. Scheduled follow-up after a pediatric emergency department visit for asthma: a randomized trial. Pediatrics. 2003;111(3):495–502. [PubMed: 12612227]
- Section 5, Managing Exacerbations of Asthma - Expert Panel Report 3: Guidelines ...Section 5, Managing Exacerbations of Asthma - Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma
Your browsing activity is empty.
Activity recording is turned off.
See more...