NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-.
This PDQ cancer information summary has current information about the treatment of myelodysplastic syndromes. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Date Last Modified") is the date of the most recent change. The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board.
General Information About Myelodysplastic Syndromes
Key Points for This Section
Myelodysplastic syndromes are a group of cancers in which immature blood cells in the bone marrow do not mature or become healthy blood cells.
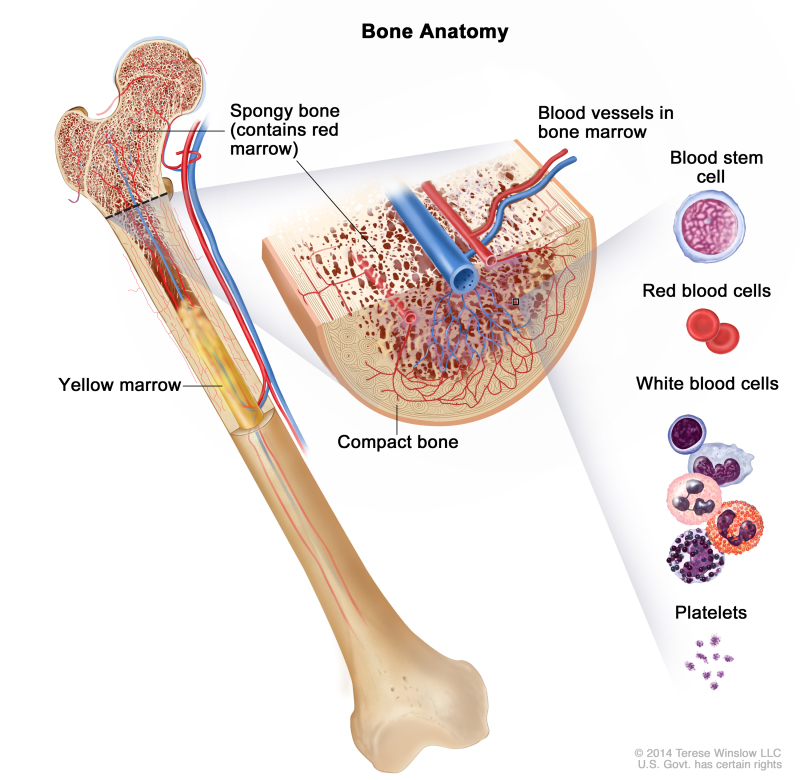
In a healthy person, the bone marrow makes blood stem cells (immature cells) that become mature blood cells over time.
Anatomy of the bone. The bone is made up of compact bone, spongy bone, and bone marrow. Compact bone makes up the outer layer of the bone. Spongy bone is found mostly at the ends of bones and contains red marrow. Bone marrow is found in the center of most bones and has many blood vessels. There are two types of bone marrow: red and yellow. Red marrow contains blood stem cells that can become red blood cells, white blood cells, or platelets. Yellow marrow is made mostly of fat.
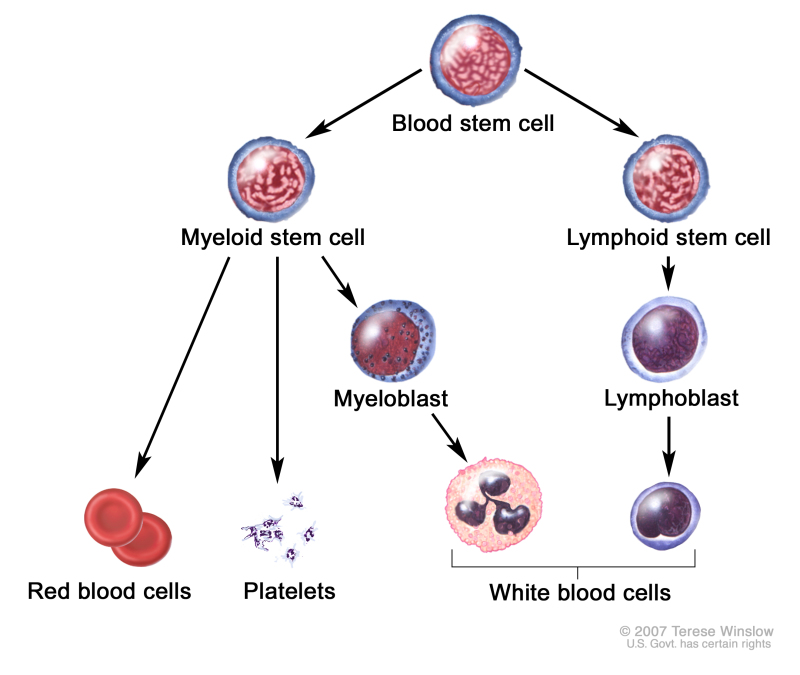
A blood stem cell may become a lymphoid stem cell or a myeloid stem cell. A lymphoid stem cell becomes a white blood cell. A myeloid stem cell becomes one of three types of mature blood cells:
- Platelets that form blood clots to stop bleeding.
- White blood cells that fight infection and disease.
Blood cell development. A blood stem cell goes through several steps to become a red blood cell, platelet, or white blood cell.
In a patient with a myelodysplastic syndrome, the blood stem cells (immature cells) do not become mature red blood cells, white blood cells, or platelets in the bone marrow. These immature blood cells, called blasts, do not work the way they should and either die in the bone marrow or soon after they go into the blood. This leaves less room for healthy white blood cells, red blood cells, and platelets to form in the bone marrow. When there are fewer healthy blood cells, infection, anemia, or easy bleeding may occur.
The different types of myelodysplastic syndromes are diagnosed based on certain changes in the blood cells and bone marrow.
- Refractory anemia: There are too few red blood cells in the blood and the patient has anemia. The number of white blood cells and platelets is normal.
- Refractory anemia with ring sideroblasts: There are too few red blood cells in the blood and the patient has anemia. The red blood cells have too much iron inside the cell. The number of white blood cells and platelets is normal.
- Refractory anemia with excess blasts: There are too few red blood cells in the blood and the patient has anemia. Five percent to 19% of the cells in the bone marrow are blasts. There also may be changes to the white blood cells and platelets. Refractory anemia with excess blasts may progress to acute myeloid leukemia (AML). See the PDQ Adult Acute Myeloid Leukemia Treatment summary for more information.
- Refractory cytopenia with multilineage dysplasia: There are too few of at least two types of blood cells (red blood cells, platelets, or white blood cells). Less than 5% of the cells in the bone marrow are blasts and less than 1% of the cells in the blood are blasts. If red blood cells are affected, they may have extra iron. Refractory cytopenia may progress to acute myeloid leukemia (AML).
- Refractory cytopenia with unilineage dysplasia: There are too few of one type of blood cell (red blood cells, platelets, or white blood cells). There are changes in 10% or more of two other types of blood cells. Less than 5% of the cells in the bone marrow are blasts and less than 1% of the cells in the blood are blasts.
- Unclassifiable myelodysplastic syndrome: The numbers of blasts in the bone marrow and blood are normal, and the disease is not one of the other myelodysplastic syndromes.
- Myelodysplastic syndrome associated with an isolated del(5q) chromosome abnormality: There are too few red blood cells in the blood and the patient has anemia. Less than 5% of the cells in the bone marrow and blood are blasts. There is a specific change in the chromosome.
- Chronic myelomonocytic leukemia (CMML): See the PDQ summary on Myelodysplastic/ Myeloproliferative Neoplasms Treatment for more information.
Age and past treatment with chemotherapy or radiation therapy affect the risk of a myelodysplastic syndrome.
Anything that increases your risk of getting a disease is called a risk factor. Having a risk factor does not mean that you will get a disease; not having risk factors doesn’t mean that you will not get a disease. Talk with your doctor if you think you may be at risk. Risk factors for myelodysplastic syndromes include the following:
- Being exposed to certain chemicals, including tobacco smoke, pesticides, fertilizers, and solvents such as benzene.
- Being exposed to heavy metals, such as mercury or lead.
The cause of myelodysplastic syndromes in most patients is not known.
Signs and symptoms of a myelodysplastic syndrome include shortness of breath and feeling tired.
Myelodysplastic syndromes often do not cause early signs or symptoms. They may be found during a routine blood test. Signs and symptoms may be caused by myelodysplastic syndromes or by other conditions. Check with your doctor if you have any of the following:
- Shortness of breath.
- Weakness or feeling tired.
- Having skin that is paler than usual.
- Easy bruising or bleeding.
- Petechiae (flat, pinpoint spots under the skin caused by bleeding).
Tests that examine the blood and bone marrow are used to detect (find) and diagnose myelodysplastic syndromes.
The following tests and procedures may be used:
- Physical exam and history: An exam of the body to check general signs of health, including checking for signs of disease, such as lumps or anything else that seems unusual. A history of the patient’s health habits and past illnesses and treatments will also be taken.
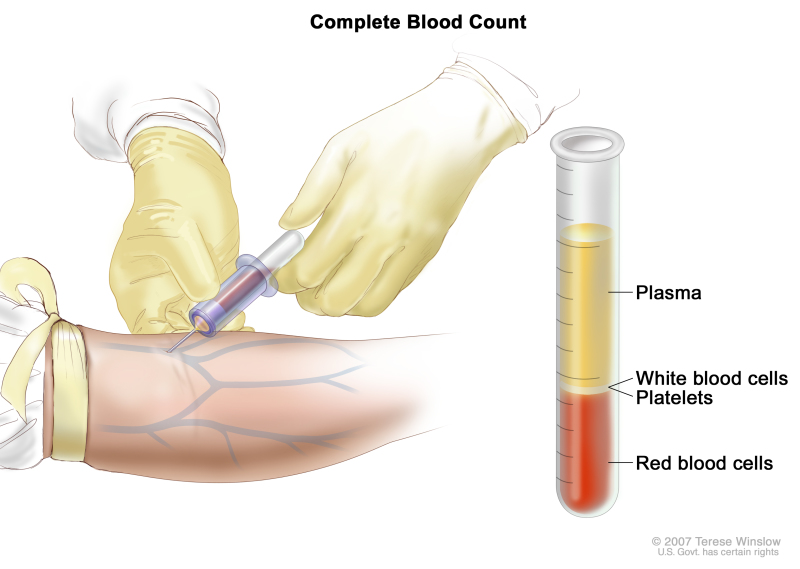
- Complete blood count (CBC) with differential: A procedure in which a sample of blood is drawn and checked for the following:
- -
The number of red blood cells and platelets.
- -
The number and type of white blood cells.
- -
The amount of hemoglobin (the protein that carries oxygen) in the red blood cells.
- -
The portion of the blood sample made up of red blood cells.
Complete blood count (CBC). Blood is collected by inserting a needle into a vein and allowing the blood to flow into a tube. The blood sample is sent to the laboratory and the red blood cells, white blood cells, and platelets are counted. The CBC is used to test for, diagnose, and monitor many different conditions.
- Peripheral blood smear: A procedure in which a sample of blood is checked for changes in the number, type, shape, and size of blood cells and for too much iron in the red blood cells.
- Cytogenetic analysis: A test in which cells in a sample of blood or bone marrow are viewed under a microscope to look for certain changes in the chromosomes.
- Blood chemistry studies: A procedure in which a blood sample is checked to measure the amounts of certain substances, such as vitamin B12 and folate, released into the blood by organs and tissues in the body. An unusual (higher or lower than normal) amount of a substance can be a sign of disease.
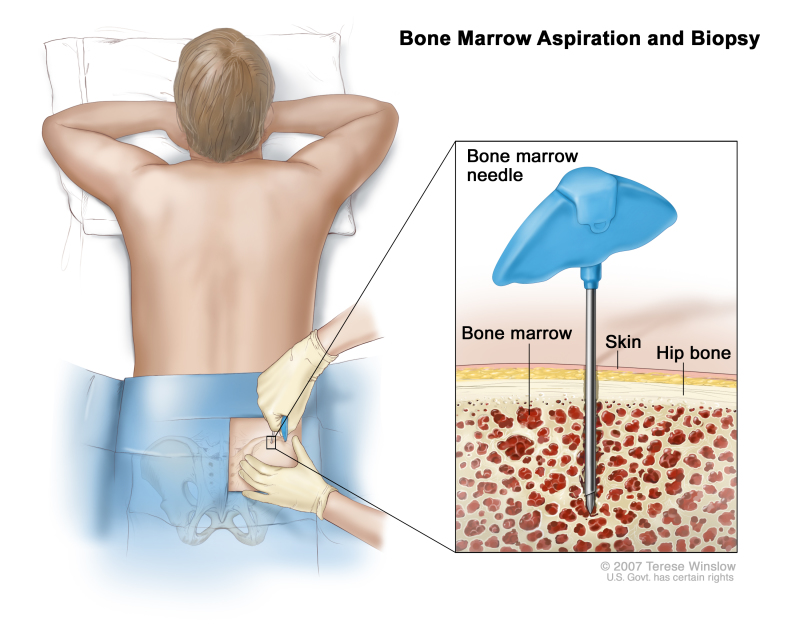
- Bone marrow aspiration and biopsy: The removal of bone marrow, blood, and a small piece of bone by inserting a hollow needle into the hipbone or breastbone. A pathologist views the bone marrow, blood, and bone under a microscope to look for abnormal cells.The following tests may be done on the sample of tissue that is removed:
- Immunocytochemistry: A test that uses antibodies to check for certain antigens in a sample of bone marrow. This type of test is used to tell the difference between myelodysplastic syndromes, leukemia, and other conditions.
- Immunophenotyping: A process used to identify cells, based on the types of antigens or markers on the surface of the cell. This process is used to diagnose specific types of leukemia and other blood disorders by comparing the cancer cells to normal cells of the immune system.
- Flow cytometry: A laboratory test that measures the number of cells in a sample, the percentage of live cells in a sample, and certain characteristics of cells, such as size, shape, and the presence of tumor markers on the cell surface. The cells are stained with a light-sensitive dye, placed in a fluid, and passed in a stream before a laser or other type of light. The measurements are based on how the light-sensitive dye reacts to the light.
- FISH (fluorescence in situ hybridization): A laboratory technique used to look at genes or chromosomes in cells and tissues. Pieces of DNA that contain a fluorescent dye are made in the laboratory and added to cells or tissues on a glass slide. When these pieces of DNA bind to specific genes or areas of chromosomes on the slide, they light up when viewed under a microscope with a special light.
Certain factors affect prognosis and treatment options.
The prognosis (chance of recovery) and treatment options depend on the following:
- The number of blast cells in the bone marrow.
- Whether one or more types of blood cells are affected.
- Whether the patient has signs or symptoms of anemia, bleeding, or infection.
- Whether the patient has a low or high risk of leukemia.
- Certain changes in the chromosomes.
- Whether the myelodysplastic syndrome occurred after chemotherapy or radiation therapy for cancer.
- The age and general health of the patient.
Treatment Option Overview
Key Points for This Section
There are different types of treatment for patients with myelodysplastic syndromes.
Different types of treatment are available for patients with myelodysplastic syndromes. Some treatments are standard (the currently used treatment), and some are being tested in clinical trials. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment. Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Treatment for myelodysplastic syndromes includes supportive care, drug therapy, and stem cell transplantation.
Patients with a myelodysplastic syndrome who have symptoms caused by low blood counts are given supportive care to relieve symptoms and improve quality of life. Drug therapy may be used to slow progression of the disease. Certain patients can be cured with aggressive treatment with chemotherapy followed by stem cell transplant using stem cells from a donor.
Three types of standard treatment are used:
Supportive care
Supportive care is given to lessen the problems caused by the disease or its treatment. Supportive care may include the following:
- Transfusion therapyTransfusion therapy (blood transfusion) is a method of giving red blood cells, white blood cells, or platelets to replace blood cells destroyed by disease or treatment. A red blood cell transfusion is given when the red blood cell count is low and signs or symptoms of anemia, such as shortness of breath or feeling very tired, occur. A platelet transfusion is usually given when the patient is bleeding, is having a procedure that may cause bleeding, or when the platelet count is very low.
- Erythropoiesis-stimulating agentsErythropoiesis-stimulating agents (ESAs) may be given to increase the number of mature red blood cells made by the body and to lessen the effects of anemia. Sometimes granulocyte colony-stimulating factor (G-CSF) is given with ESAs to help the treatment work better.
- Antibiotic therapyAntibiotics may be given to fight infection.
Drug therapy
- LenalidomidePatients with myelodysplastic syndrome associated with an isolated del(5q) chromosome abnormality who need frequent red blood cell transfusions may be treated with lenalidomide. Lenalidomide is used to lessen the need for red blood cell transfusions.
- Immunosuppressive therapyAntithymocyte globulin (ATG) works to suppress or weaken the immune system. It is used to lessen the need for red blood cell transfusions.
- Azacitidine and decitabineAzacitidine and decitabine are used to treat myelodysplastic syndromes by killing cells that are dividing rapidly. They also help genes that are involved in cell growth to work the way they should. Treatment with azacitidine and decitabine may slow the progression of myelodysplastic syndromes to acute myeloid leukemia.
- Chemotherapy used in acute myeloid leukemia (AML)Patients with a myelodysplastic syndrome and a high number of blasts in their bone marrow have a high risk of acute leukemia. They may be treated with the same chemotherapy regimen used in patients with acute myeloid leukemia.
Chemotherapy with stem cell transplant
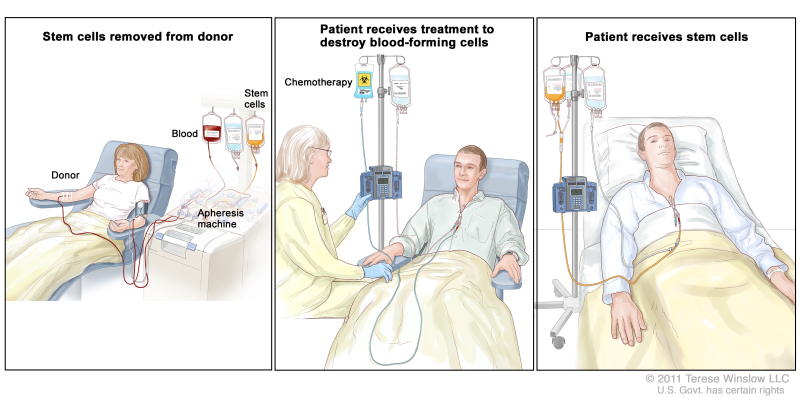
Stem cell transplant is a method of giving chemotherapy and replacing blood-forming cells destroyed by the treatment. Stem cells (immature blood cells) are removed from the blood or bone marrow of a donor and are frozen for storage. After the chemotherapy is completed, the stored stem cells are thawed and given back to the patient through an infusion. These reinfused stem cells grow into (and restore) the body's blood cells.
This treatment may not work as well in patients whose myelodysplastic syndrome was caused by past treatment for cancer.
Stem cell transplant. (Step 1): Blood is taken from a vein in the arm of the donor. The patient or another person may be the donor. The blood flows through a machine that removes the stem cells. Then the blood is returned to the donor through a vein in the other arm. (Step 2): The patient receives chemotherapy to kill blood-forming cells. The patient may receive radiation therapy (not shown). (Step 3): The patient receives stem cells through a catheter placed into a blood vessel in the chest.
New types of treatment are being tested in clinical trials.
Information about clinical trials is available from the NCI website.
Patients may want to think about taking part in a clinical trial.
For some patients, taking part in a clinical trial may be the best treatment choice. Clinical trials are part of the cancer research process. Clinical trials are done to find out if new cancer treatments are safe and effective or better than the standard treatment.
Many of today's standard treatments for cancer are based on earlier clinical trials. Patients who take part in a clinical trial may receive the standard treatment or be among the first to receive a new treatment.
Patients who take part in clinical trials also help improve the way cancer will be treated in the future. Even when clinical trials do not lead to effective new treatments, they often answer important questions and help move research forward.
Patients can enter clinical trials before, during, or after starting their treatment.
Some clinical trials only include patients who have not yet received treatment. Other trials test treatments for patients whose cancer has not gotten better. There are also clinical trials that test new ways to stop cancer from recurring (coming back) or reduce the side effects of cancer treatment.
Clinical trials are taking place in many parts of the country. Information about clinical trials supported by NCI can be found on NCI’s clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Follow-up tests may be needed.
Some of the tests that were done to diagnose the cancer or to find out the stage of the cancer may be repeated. Some tests will be repeated in order to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your condition has changed or if the cancer has recurred (come back). These tests are sometimes called follow-up tests or check-ups.
Treatment Options for Myelodysplastic Syndromes
Standard Treatment Options for Myelodysplastic Syndromes
Standard treatment options for myelodysplastic syndromes include:
- Supportive care with one or more of the following:
- Antibiotic therapy.
- Treatments to slow progression to acute myeloid leukemia (AML):
- Azacitidine and decitabine.
- Chemotherapy used in acute myeloid leukemia.
- Chemotherapy with stem cell transplant.
Treatment of Therapy-Related Myeloid Neoplasms
Patients who were treated in the past with chemotherapy or radiation therapy may develop myeloid neoplasms related to that therapy. Treatment options are the same as for other myelodysplastic syndromes.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Treatment Options for Relapsed or Refractory Myelodysplastic Syndromes
There is no standard treatment for refractory or relapsed myelodysplastic syndromes. Patients whose cancer does not respond to treatment or has come back after treatment may want to take part in a clinical trial.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
To Learn More About Myelodysplastic Syndromes
For more information from the National Cancer Institute about myelodysplastic syndromes, see the following:
For general cancer information and other resources from the National Cancer Institute, see the following:
About This PDQ Summary
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about the treatment of myelodysplastic syndromes. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Date Last Modified") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials are listed in PDQ and can be found online at NCI's website. Many cancer doctors who take part in clinical trials are also listed in PDQ. For more information, call the Cancer Information Service 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Myelodysplastic Syndromes Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/myeloproliferative/patient/myelodysplastic-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389239]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 2,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us.