This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
Guidelines urge primary care practices to provide routine tobacco-cessation care. Implementation of effective and sustainable strategies is lacking, especially for socially and economically disadvantaged populations. We tested a systems-based approach that engages the medical assistant (MA) who records the patient's vital signs at the beginning of a routine visit and the added effect of a clinician-based approach that draws on a relationship-centered communication strategy.
Objectives:
This project aimed to (1) improve delivery and documentation of tobacco-cessation care to disadvantaged patients using an Ask-Advise-Connect (AAC) systems-based approach; (2) test the effect of combining the clinician-based Teachable Moment Communication Process (TMCP) intervention with AAC on advice to quit, referrals to cessation counseling, and provision of tobacco-cessation medications; and (3) examine the narratives of patient subgroups to understand and improve the referral experience.
Methods:
This study engaged a health care system and 8 primary care clinical sites with 2 interventions. The 3-month period before the AAC intervention represented a pre-AAC control period (baseline). All sites received the AAC strategy throughout the study, and its use was evaluated for a minimum of 6 months (AAC only). Next, using a group-randomized, stepped-wedge design, sites received the TMCP intervention (AAC+TMCP). The patient population consisted of 40% Medicaid, 23.9% Medicare, 6.1% uninsured, and 30% commercially insured individuals. The AAC strategy involved changes to the electronic health record (EHR), a new role for MAs, and a new capacity to send electronic referrals to the quitline (QL) to enroll patients in tobacco-cessation counseling. Next, in accord with their practice's place in the stepped-wedge design, 44 of the 60 eligible clinicians attended training on the TMCP, an approach to counseling patients to quit tobacco that is aligned with patient readiness. Generalized linear models tested the effect of interventions on immediate outcome measures of process, including delivery of advice, offers of assistance and referrals accepted, and QL contact and enrollment rates. Receipt of tobacco-cessation medications and quit attempts were also assessed. The primary outcome was QL contact. In-depth interviews were conducted with 55 patients referred to the QL to explore their experiences and identify opportunities to improve the referral process.
Results:
Of the 224 079 visits to 1 of the 8 clinical sites during the study period, 37 909 (25.9%) were made by identified tobacco users.
Effect of AAC:
All indicators of AAC use significantly increased post implementation. Compared with the pre-AAC period, the following process measures increased and remained significant 12 months post-AAC: assessing smoking status (26.6% vs 55.7%; odds ratio [OR], 3.7; 95% CI, 3.6-3.9); providing advice (44.8% vs 88.7%; OR, 7.8; 95% CI, 6.6-9.1); assessing readiness to quit (15.8% vs 55.0%; OR, 6.2; 95% CI, 5.4-7.0); and acceptance of referral to tobacco-cessation counseling (0.5% vs 30.9%; OR, 81.0; 95% CI, 11.4-575.8). This process generated 1223 QL referrals; 324 (31.1%) patients were contacted by the QL, 241 (74.4%) were enrolled, and 195 (80.9% of enrollees) completed at least 1 counseling session.
Effect of TMCP:
In total, 44 of 60 eligible clinicians received the TMCP training. During the 6-month post-TMCP intervention period, 68% of TMCP-trained clinicians used a TMCP approach (documented by flow sheet use) ≥1 times, with the median number of uses being 15 (interquartile range, 2-33). Overall, the TMCP was used in 661 of 8198 visits by smokers (8%). There was no improvement in any of the outcomes for the AAC+TMCP group vs the AAC-only group. Among visits when clinicians used the TMCP approach, there was a significant increase in the ordering of tobacco-cessation medications (OR, 2.6; 95% CI, 1.9-3.5). Provision of brief advice, assessment of readiness to quit, contact by and enrollment in the QL program, and quit attempts did not improve. Among a subsample of approximately 125 patients per time period, there were no adverse effects on visit satisfaction for either the AAC-only or the AAC+TMCP intervention time periods compared with the pre-AAC time period. Analysis of in-depth interviews with participants initially agreeing to QL contact found that the major barriers preventing patients from completing the QL program included lack of clear expectations for the QL, life stressors preventing enrollment, and difficulty making time for the counseling sessions. Regardless of level of engagement with the QL program, patients encouraged primary care teams to continue asking them about their smoking status and offering tobacco-cessation support.
Conclusions:
The AAC system change intervention substantially increased the provision of tobacco-cessation care, with improvements sustained beyond 1 year. Adding TMCP training for clinicians improved ordering of tobacco-cessation medications, but other outcomes did not improve. Future work requires more complete integration of the AAC and TMCP approaches and tools into EHR systems for the combined process to be fully tested.
Limitations:
The study was conducted in 1 health care system with a single EHR system. The modest uptake of the TMCP approach after training (8% of smokers' clinic visits) limited the ability to assess the intervention's impact.
Background
Smoking is the leading cause of preventable mortality. Smoking rates in the United States have declined but remain particularly high among socially and economically disadvantaged populations, who are also less likely to use support methods for quitting smoking.1-5 Therefore, implementing and evaluating strategies that work for socially and economically disadvantaged populations is a high priority. Among US smokers, 59% report seeing a primary care clinician at least yearly,6 making primary care a major avenue for providing tobacco-cessation assistance tailored to each patient's medical history. However, evidence-based interventions that address tobacco cessation are generally underused.7 New health care policy initiatives that require assessment of tobacco use and offer assistance to quit require the integration of tobacco-cessation interventions into primary care.8
Referral to quitlines (QLs) that provide evidence-based, effective, and cost-efficient assistance with smoking cessation5,9-12 is recommended as an effective strategy for providing tobacco-cessation assistance.13-17 However, a clinician's recommendation to call a QL typically results in poor calling rates (range, 1.6%-19%).18 Success rates do increase when QLs proactively call patients after receiving a direct clinician referral (by fax or electronically).19-23 Still, clinicians underuse direct referrals,24 potentially because of low rates of integrating the referral system into the office workflow.24 Research demonstrates the feasibility of closed-loop electronic referrals using electronic health records (EHRs)25 for connecting with QLs. The advantage of e-referral is that patient information is securely transmitted between the referring clinician and the QL, and QL enrollment and counseling completion records become part of the patient medical record.
Two intervention approaches for providing patients with assistance for smoking cessation have shown promise in the primary care setting. Ask-Advise-Connect (AAC) is a systems-based approach that uses the EHR to remind clinicians to ask about tobacco use and give advice to quit, and then electronically connects interested smokers to tobacco-cessation counseling services, such as the QL. The Teachable Moment Communication Process (TMCP) is a relationship- and communication-focused strategy designed to counsel patients about behavior change by responding in a way both appropriate to and aligned with the patient's readiness for a change.26-29 These approaches are feasible and complementary, and they can potentially be integrated into a health system to improve sustainability.
Ask-Advise-Connect
This proactive, direct-messaging EHR-to-QL referral approach has been shown to increase the number of tobacco users receiving QL treatment 13- to 30-fold.30,31 However, a major drawback of AAC is a low rate of contacting referred patients. Earlier studies found that the QL was able to contact <42% of referred patients. This poor contact rate diminishes the sustainability of the approach and is likely the result of referring patients inappropriately, ignoring cessation readiness.25,32,33 In this study, we assessed the patient's readiness to quit and willingness to be connected to the QL before making an e-referral, thereby ensuring appropriate referrals. A drawback of some studies of AAC implementation strategies is that they rely on study research staff to carry out a key step30,31 rather than using a design that is fully integrated and sustainable in clinical practice. In this study, we trained medical assistants (MAs) and nurses to implement the AAC and to modify EHR functionality to facilitate an e-referral, thereby providing a more sustainable approach.
Teachable Moment Communication Process
With the TMCP, the discussion about smoking is initiated in an opportunistic way so that it fits into the flow of addressing concerns during a primary care visit. In providing tobacco-cessation advice, the TMCP calls for clinicians to convey concern, express optimism and partnership, and recommend quitting tobacco. Finally, central to this approach is eliciting an honest assessment of the patient's level of cessation readiness and responding in alignment with this readiness. Research shows that the TMCP approach increases patient motivation to make a quit attempt. Evidence is accumulating that the TMCP intervention is feasible in primary care and acceptable to patients and clinicians, leading to significant increases in clinicians' use of recommended counseling behaviors and activating patients during primary care visits.27,29,34,35
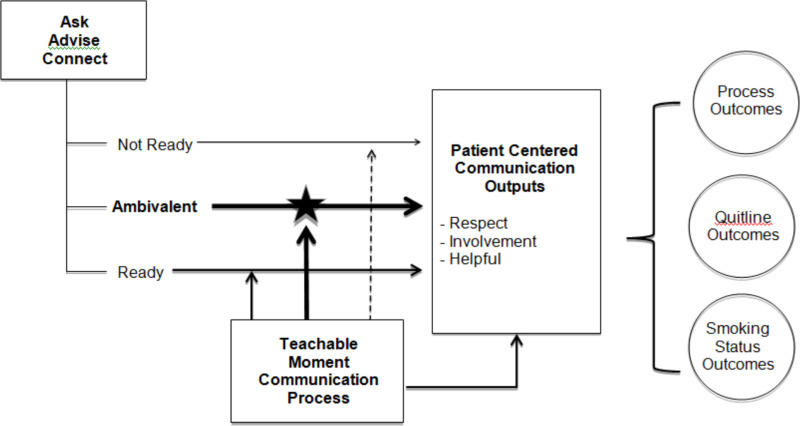
We propose that the AAC and TMCP interventions have mutually complementary strengths and that their combined implementation may synergistically boost positive outcomes.36 The AAC intervention is designed to be deployed at every visit so that all patients are assessed for smoking status, advised to quit, assessed for their readiness to quit, and offered assistance. Because it is a part of every office visit, the AAC can reach a large percentage of patients. The TMCP intervention, in contrast, is situation specific and driven by identification of and clinician action on a salient concern that arises during the visit. By accounting for concerns salient to the patient, the TMCP can help patients move forward along the readiness-to-quit continuum. It also reinforces the value of quitting by incorporating clinician advice to quit, which has been shown to increase quit rates.22 If implemented together, these 2 interventions could have a synergistic effect (see Figure 1) whereby the interventions operate at different levels of influence to mutually reinforce each other. Their combined effect has great potential to increase the proportion of appropriate referrals (ie, eligible and ready patients) to the QL, increase the likelihood of successful patient contact and enrollment, positively affect patients' ratings of the experience, and support positive movement toward cessation.
The study aims were as follows:
- Improve delivery and documentation of tobacco-cessation advice and assistance to socially and economically disadvantaged patients using an AAC approach integrated into the EHR
- Test the effect of combining the TMCP with the AAC on process outcomes, QL referral outcomes, and smoking cessation outcomes
- Examine the narratives of subgroups of individuals to better understand the referral experience and identify ways to improve it
Patient and Stakeholder Engagement
Key stakeholders for this project included primary care clinicians and staff; health care system administrators, representing primary care and information systems (IS); tobacco-cessation service providers; Ohio Department of Health Tobacco Use Prevention and Cessation program representatives; Ohio QL operations representatives; and patients who use tobacco.
Identifying Stakeholders
Our project's strong partnership with MetroHealth was critical for designing and implementing both interventions. The MetroHealth System is a safety-net health system in Cuyahoga County, which includes Cleveland, Ohio, and serves the largest portion of Medicaid and uninsured patients in the region. Approximately 75% of outpatient visits are by individuals who are uninsured or have Medicaid or Medicare insurance. Among residents of Cleveland, 35% live below the poverty line.37 In 2017, 30% of MetroHealth primary care patients reported using tobacco. MetroHealth has exceptional capacity to use EHRs for quality improvement and system change, and it has been recognized since 2014 by the Healthcare Information and Management Systems Society as a stage-7 EHR Ambulatory Adoption Model health care system in all its ambulatory clinical sites and hospitals. Stage 7 represents the highest level of EHR adoption and use and indicates the health system's advanced EHR implementation. MetroHealth's culture of supporting quality improvement efforts facilitated our ability to embed the interventions into existing workflows, thereby improving sustained implementation.
Several stakeholders, including clinicians (n = 2) and representatives from the Ohio Department of Health (n = 1) and Ohio QL (n = 2) were engaged in the project from conception by invitation from the lead investigators. We recruited 2 patient stakeholders from among individuals who had recently quit smoking as well as former smokers identified by MetroHealth staff members who led an in-house tobacco-cessation program. Patient representatives helped shape our approach to primary data collection and measures on the patient survey. Other stakeholders engaged throughout the study included MAs (n = 3), nurses (n = 2), nurse managers (n = 2), informatics representatives (n = 2), IS representatives (n = 1), and Epic software trainers (n = 2). These roles and individuals were identified by team members and other stakeholders as important perspectives for engaging in the study at key junctures in the study timeline and as we collectively began to understand the practice operations, challenges, and opportunities presented by implementing the study. The study leader worked to foster an appreciation across the group that all stakeholder voices were important to informing and guiding our project.
Engagement Activities
We conducted quarterly in-person stakeholder meetings. The meeting location accommodated the majority of the stakeholder group. Phone and video conferencing options were used for stakeholders who needed to travel significant distances to attend the meetings (eg, QL representatives). To increase time efficiency, meeting agendas and important materials were shared in advance to enable all stakeholders to prepare and fully engage in discussions.
In the beginning, we developed and agreed on guidelines to ensure that all stakeholders felt valued and knew that their ideas mattered regardless of degrees or status. For example, to work with groups in which some members had little research experience, we introduced “jargon-busting” methods of explanation paired with encouraging, clarifying questions. We also provided orientations to the project goals, emphasizing the meaning and importance of the project to each stakeholder. Other examples included encouraging active and respectful listening, embracing differing opinions, and keeping an open mind about new ideas. All participants were equal partners and received the same stipend for participation in the quarterly meetings.
Stakeholder Impact
Input from our stakeholders helped define several key aspects of the project. Specifically, the health system representatives influenced the decision to include patient subgroups for in-depth interviews and recommended inclusion of all clinicians in the TMCP training using the stepped-wedge design. Patient stakeholders' experiences were vital for designing the project's survey and streamlining the process for inviting participants to complete a survey. Further, input from MAs and nurses boosted AAC development, including critical upgrades to training, learning materials (eg, TipSheet), and EHR design. Stakeholder group representatives also helped identify people within organizations whose input at different stages of the project was crucial to the development and success of the project. Finally, stakeholders' feedback about early findings helped chart the next steps for our inquiry.
In addition to providing impactful input to the project, stakeholders demonstrated significant interest in the outcomes and activities resulting from the study. Specifically, our stakeholders from MetroHealth announced their plans for incorporating AAC into the system wide flow for all clinic specialties. Further, MetroHealth representatives plan to implement the infrastructure developed during the study to send secure e-referrals from the EHR to QL services and to use it as a prototype for providing EHR referrals to external services. Finally, our stakeholders from QL services asked for qualitative findings from in-depth interviews to inform their efforts in making QL processes more user friendly.
Methods
Study Overview
For this cluster randomized controlled trial (RCT) with a stepped-wedge design, we compared tobacco-cessation support provided during primary care clinic visits between 3 periods: (1) pre-AAC baseline; (2) AAC only; and (3) AAC+TMCP training, in which both AAC and TMCP were implemented. The primary outcome was QL contact rate. Other study outcomes were measures of process, including delivery of advice; offer of assistance and referrals accepted; and QL enrollment rates. The cluster RCT is the best choice for this study because patient visits are the unit of analysis clustered within sites and clinicians; further, the 2 interventions were deployed at the site and clinician levels. Because there was a relatively small number of practice sites to work with and because the health system stipulated that all sites eventually receive the intervention rather than serving only as control sites, we used a stepped-wedge design. In such a design, all sites begin in the control condition and each site is randomly assigned to a time point when it will receive the intervention. This design has greater statistical power over a traditional 2-group RCT.38
The intervention phase began with a study site implementing AAC. All sites implemented AAC at the beginning of the intervention phase. After a period of observing the effects of AAC alone, the study team conducted the TMCP training for each site at the beginning of the site's randomly assigned date to implement TMCP. The site was asked to start using the TMCP approach immediately in routine patient care. We examined the impact of the effect of the TMCP approach in 3 ways: intent to treat (by including all clinicians at the site), per protocol (by including those clinicians exposed to the TMCP training), and per documented use in a visit (indicated by the EHR document flow sheet developed for this study; document flow sheets are EHR templates for gathering and documenting information during an encounter). Table 1 outlines the key features of each intervention, the anticipated influences, the possible reach, and the outputs of the intervention. Immediate study outcomes were measures of process, including delivery of advice; offer of assistance and referrals accepted; and QL contact and enrollment rates. Additionally, we evaluated specific patients' clinic experience by surveying them and their QL referral process experience by conducting in-depth interviews.
Study Setting
The study was conducted in 8 primary care community health centers within the MetroHealth System in Cleveland. The rationale for selecting the MetroHealth System was 3-fold: It serves the largest portion of Medicaid and uninsured patients in the region; a high proportion of its patients use tobacco; and it has the capacity to use the Epic EHR system to implement quality improvement initiatives.
Participants
Patient participants were not specifically recruited for this study. Instead, all adult patients (defined as aged ≥18 years) coming for primary care visits to the study-linked clinics during the study period contributed their EHR data to analyses for aims 1 and 2. The IRB waived patient informed consent for use of the EHR data for 3 reasons: (1) the large number of patients, which made obtaining individual consent impracticable within the time frame of the study; (2) the plan to use deidentified data in the analytic data file; and (3) the overall low-risk nature of study participation. All MAs and registered nurses (RNs) were asked to attend the AAC training. All clinicians at each of the 8 study sites were asked by the health care system to participate in the TMCP training; they were not study volunteers. For the process variables, the unit of analysis is the patient visit. Clustering by clinician and by site is accounted for in our analyses.
Study Design and Timing
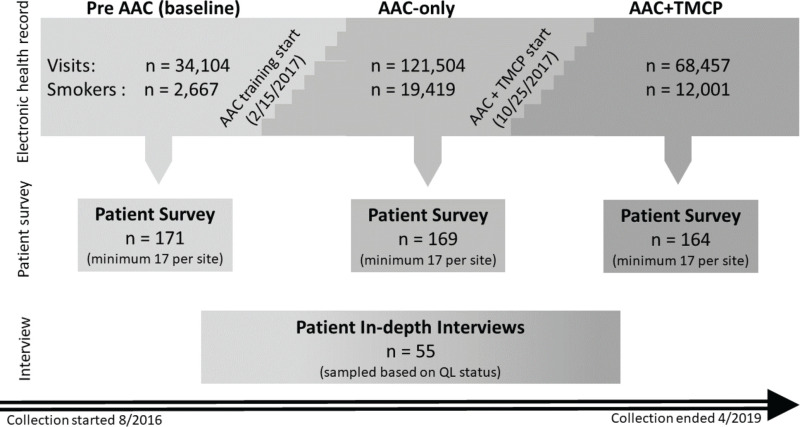
This study involved 2 interventions. The AAC intervention was conducted first at the clinic level; the TMCP intervention was conducted second at the clinician level. The study also included qualitative data collection to complement the quantitative evaluations. Figure 2 illustrates the 3 main data collection sources (EHR, patient surveys, and in-depth interviews) and the 3 periods of data collection (pre-AAC, AAC only, and AAC+TMCP). The 3-month period before the AAC intervention represented a pre-AAC control period (baseline). Both interventions were implemented using a stepped-wedge design, as indicated in Figure 2. The start date represents the date of the first clinic's exposure to the interventions. The step graphic between each condition represents the starting points for each clinic's exposure to the interventions. This design enabled us to test the additional impact of the TMCP approach beyond the gains realized with AAC across all the clinics by the end of the stepped-wedge implementation. On a rolling basis, data collection indicated the intervention exposure statuses for each clinic and assessed visit-level process outcomes.
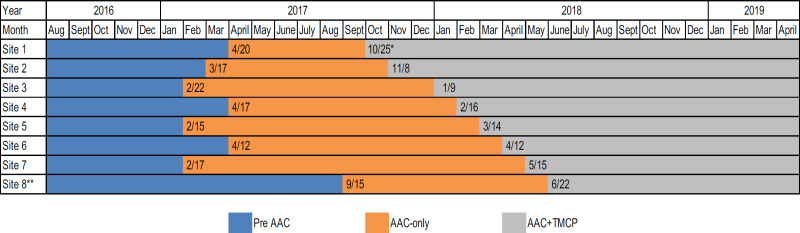
Figure 3 provides more detail about the timing of the AAC and the TMCP intervention implementations at each of the 8 sites. AAC was the first intervention implemented, and the AAC training at the first clinic was conducted on February 15, 2017. This training was then conducted at the other 7 clinics at approximately 2-week intervals, but the timing of the training was not randomized. Trainings were scheduled during regularly scheduled monthly staff meetings for each clinic. The training date was noted such that a period of pretraining and a period of posttraining could be defined for each clinic. Approximately 6 months after the implementation of AAC in the last site, the TMCP intervention was implemented, again in a stepped pattern.
For the TMCP intervention, the 8 clinics were randomized to an implementation time point using the following approach. A single-digit number was assigned to each site. A list of 100 random single-digit numbers was generated; using this list, as a site number came up in the random list, it was assigned to the next sequential time point. We encountered 2 anomalies with randomization of the sites. First, after implementing the AAC intervention, the site that was randomized to receive the TMCP training lost its practice manager. Because of challenges with daily operations at that site, MetroHealth requested that we delay the implementation of TMCP until a new practice manager was effectively in place. This site was moved to be last. Second, also after the implementation of the AAC intervention, 1 of the sites closed. A replacement site was identified to receive the AAC intervention and was included as the last site for training for the TMCP approach. The TMCP implementation time points were scheduled as close to 1-month intervals as possible. The first clinic received the TMCP training on October 25, 2017. The amount of time a clinic was in any 1 of the 3 defined study conditions varied but is accounted for in the analyses.
For aims 1 and 2, in addition to collecting EHR data from all patients aged ≥18 years, we drew a random sample to survey patients' experiences with the clinic's process. The sample included at least 17 smokers from each of the 8 sites from each study period (pre-AAC, AAC only, AAC+TMCP). The survey was collected from patient subsamples across the 8 sites during 3 periods: pre-AAC, AAC only, and AAC+TMCP implementation. Patients who attended a primary care visit during specified dates were invited to complete the survey. The survey was completed by web or phone, depending on the participant's preferred contact method. Participation was voluntary.
For aim 3, to conduct in-depth interviews, 3 subgroups provided narratives about QL experience and guidance on process improvement. Groups included patients referred to QL who (1) declined participation in the QL, (2) were determined to be unreachable by the QL, or (3) completed the QL program of 5 counseling sessions.
Interventions: Comparators and Controls
The AAC and TMCP were the 2 comparator interventions. The rationale for comparing the AAC alone with AAC and TMCP combined was to determine whether the patient-centered TMCP approach can boost patient outcomes beyond the benefits of using AAC alone. Appendices 1 and 2 describe the interventions and the implementation strategy for each in detail. Exposure to the interventions was at the clinic level (AAC) and physician level (TMCP). Assessments of effect on process and patient outcome variables began immediately after implementation and continued for a minimum of 12 months for the AAC and 6 months for the TMCP interventions.
The AAC Process
To implement the AAC intervention, we implemented 3 substantial clinical care process components: (1) establishing e-referral capacity between MetroHealth and the Ohio QL; (2) revising the EHR to prompt and document discussion of tobacco use, readiness to quit, and willingness to receive a referral for tobacco-cessation assistance; and (3) updating clinic staff roles and processes through training and support in using the revised EHR. These components are described in detail in Appendix 1. The role and process changes that were the focus of the implementation training are highlighted in Figure 4. Table 2 outlines what was in place and assumptions about responsibilities for carrying out the process before and after implementation of the AAC intervention. Patient eligibility for QL services included at least 1 of the following: being aged ≥18 years with Medicaid insurance or no insurance, or being pregnant. The QL services included up to 5 telephone counseling sessions and access to web and online chat support. Individuals who were not eligible for the QL were referred to the in-system Freedom From Smoking (FFS) program, an in-person, 8-session group tobacco-cessation class offered by the health care system. The EHR automatically generated the correct referral order using patient payer data. Therefore, regardless of eligibility, the process was seamless for MAs/RNs and patients.
AAC was implemented through in-person training at each practice site using a presentation of the rationale for the process changes, the specific tasks, the changes to the EHR, and the fact that the role change was to take effect immediately. Hands-on training, with case scenarios to practice using the new EHR display fields and orders, enabled MAs and RNs to experience the process and ask further questions. Audit and feedback provided at the practice level were used to encourage and support uptake.
Teachable Moment Communication Process
Clinicians can use the TMCP approach to initiate discussion about smoking and smoking cessation by incorporating patients' salient concerns into a tailored, partnership-oriented behavior-change discussion integrated into the flow of patient care. Central to this approach is eliciting an honest self-assessment of the patient's level of cessation readiness, and then responding with assistance aligned with the patient's readiness.34 Unlike AAC, which is designed to be used at all nonurgent clinic visits, the TMCP approach was to be used strategically at the clinician's discretion during visits when the clinician felt that there was an important opportunity and sufficient time to address tobacco cessation.
For the TMCP training, our team adapted in-person training to a web-based training module that consisted of the key learning points; demonstrations of the process, with example patient scenarios; and quiz items throughout the module to promote engagement and assessment of knowledge acquisition. Skill practices, which were included in the intervention as a way to learn behavioral enactment of each skill, took place in the examination rooms at each clinic to provide a realistic setting. The skill practice involved engagement using 6 to 8 case scenarios played by standardized patients (ie, individuals recruited and trained to take on the characteristics of real patients in a simulated clinical environment); a coach observed, and then provided feedback and additional training in technique. The clinician participant had exposure to 6 to 8 scenarios played by standardized patients and up to 4 coaches, all trained in the TMCP approach. Implementation of the TMCP training was conducted at each practice at the random order–assigned intervention date. Appendix 2 provides details of the TMCP intervention.
Development of the Document Flow Sheet
A document flow sheet was developed to serve as a guide for clinicians, with steps and phases for the TMCP, and to document delivery of and patient responses to each TMCP step (see Appendix 2). The TMCP flow sheet eliminated the need for the provider to remember the steps and their order so that they could document the patient responses with minimal effort. In addition, a tobacco-cessation order set in the EHR could be used by providers to order medication and nicotine replacement and to order a referral to the QL or tobacco-cessation classes.
During the skills training, clinicians were shown how to use the TMCP document flow sheet and had it added to their Epic tool shortcuts. Clinicians used the document flow sheet during the skills practice scenarios to gain experience with the different features it allowed. The tobacco-cessation order set was also reviewed and used during skills practice.
Debriefing About the Training
Following completion of the web module and the skills practices, the clinicians regrouped with the research team to debrief about their experiences. Clinicians provided feedback on format, length of training, processes, and content, and discussed the perceived value overall.
Study Outcomes
Table 3 summarizes the sources of data and key indicators for the study. The population or sample for which the indicators were assessed is noted in the last column. Data were drawn from the EHR, QL data, patient surveys, and in-depth interviews.
Primary Outcome
The primary outcome was QL contact. Other outcomes for the overall study were (1) tobacco-cessation support documentation (eg, documentation of assessment of tobacco use in the past 30 days—namely, “Ask,” documentation of provision of brief advice [ie, “Advise”]; (2) QL enrollment; and (3) patients' evaluations of key characteristics of the provision of tobacco-cessation support in the primary care setting.
Smoking cessation support documentation
The AAC approach includes multiple steps. Each was assessed as a process outcome.
Contact and enrollment of referred patients
Outcome variables for both the QL and FFS included percentage of referred patients who accepted referral, successful contacts, patient enrollment, and percentage of enrolled patients who completed ≥1 counseling sessions.
Patient evaluation of tobacco-cessation assistance
The AAC and TMCP interventions changed the way clinicians and staff interacted with patients during office visits, and patients' experiences about these changes were evaluated in a survey. We created a measure based on our work with former smokers from our stakeholder team, our previous experience with tobacco-cessation process measures,39 and cessation literature.25,40,41 The items were reviewed by a sample of patients who pilot-tested acceptability of the survey to patients. The consent process to participate in the survey and the survey items are reported in Appendices 3 and 4, respectively.
Qualitative interviews
In-depth qualitative interviews were conducted with a subgroup of patients who agreed to be connected to QL counselors. An interview guide contained questions to elicit patient narratives about their experiences with the e-referral and QL processes and obtain their suggestions for improvement. In total, 55 patients from 3 categories participated, all connected through e-referral: (1) those who enrolled in and completed QL counseling, (2) those who declined QL counseling, and (3) those unreachable by QL after multiple unsuccessful contact attempts.
Secondary Outcomes
Two secondary outcomes were also assessed. Orders for tobacco-cessation medications included any form of nicotine replacement therapy (eg, gum, patch), varenicline, or bupropion documented in the EHR. For this study, a quit attempt was defined as a change in smoking status from current smoker to former smoker and documentation of a quit date in the EHR.
Other Variables
Potential covariates included patient sex, race, age, and type of insurance.
Sample Size Calculations and Power
We describe sample size considerations related to the surveys and to the EHR-documented outcomes separately. In each case, we provide an illustrative estimate of available power to detect an important outcome. Our intended survey sample included surveys of 170 patients after a primary care visit at a clinic before the AAC intervention, 170 more patients visiting a clinic with AAC only, and 170 additional patients visiting a clinic after the AAC+TMCP intervention. This sample size could not permit meaningful comparisons between individual clinics on survey items but did enable us to find meaningful changes in rates of satisfaction across clinics from pre-AAC to post-AAC implementation and from AAC-only to AAC+TMCP implementation. If baseline satisfaction is at 75%, for instance, we would have 90% power with this sample size to detect an improvement to 90% with AAC using a 2-sided α = 5% significance test. A similar gap from 80% to 95% baseline satisfaction after AAC would yield 97% power.
Next, we considered comparisons between AAC only and AAC+TMCP on EHR-documented outcomes—specifically, estimating our power to compare contact rates among patients agreeing to be referred to tobacco-cessation counseling. We selected this outcome because it affects a much smaller group of patients (smokers agreeing to referral) than our documentation outcomes. We assumed that approximately 25% of smokers would agree to referral, yielding at least 240 potential contacts per month referred to the QL across 8 steps (site-specific clusters of primary care providers) in our stepped-wedge design. We assumed that the intraclass correlation coefficient within each cluster would be no larger than 0.10. We also assumed that we could measure QL contact rates monthly, for at least 3 months in the AAC-only period at all sites, and again for at least 3 months after AAC+TMCP implementation at all sites (Figure 3). This design resulted in a stepped-wedge design effect63 of 0.265, meaning that the study (which accounts for clustering by step and uses repeated measures at each step) might require about a quarter of the participants needed in an individual patient-level RCT with similar goals. We projected that the AAC-only QL contact rate would be approximately 40%. We considered a 10% absolute difference to be a clinically meaningful improvement. Accounting for clustering and repeated measures through the stepped-wedge design effect, with a 2-tailed α = 5% significance level, we predicted 86% power to detect a change in QL contact rate from 40% to 50% and 99% power for a QL contact rate change from 40% to 55%.
Time Frame for the Study
We used EHR data for a 2-year (retrospective) period to develop and test our methods for data extraction and to establish methods to generate complex variables, such as quit attempts. For the active study period, a 3-month pre-AAC period of the current practice assessment (baseline) was followed by implementing AAC at all 8 practices over 3 months (starting in February 2017). The AAC-only implementation effect was evaluated for approximately 12 months. Next, the TMCP training was implemented (starting in October 2017) using the stepped-wedge design.38 The combination of AAC and TMCP was evaluated for 6 months after TMCP implementation. Figures 2 and 3 show the timing of the interventions.
Data Collection and Sources
The main data sources collected for the project were (1) EHR data assessed for all visits, (2) patient survey data from random patient subsamples at each study period, and (3) narrative data from the in-depth interviews (Figure 2).
Analytical and Statistical Approaches
Key Analytical Considerations
We compared outcomes across 3 different intervention conditions: usual care before AAC, AAC only, and AAC+TMCP. Data for adult patients with a primary care visit to 1 of the 8 clinic sites were extracted from the EHR to yield the pre-AAC data. (See Figure 3 for the depiction of the time intervals referred to here.)
Aim 1 analyses compared pre-AAC to AAC-only data across patient visits to assess improvement in delivery and documentation of tobacco-cessation advice. Descriptive statistics were reported for the samples during the pre-AAC and AAC-only intervention periods. Process variables representing the proportion of current tobacco users who were asked, advised, assessed about their readiness, and offered a referral were reported by time period. Using the 3 months before implementation as the reference, generalized estimating equations (GEEs) were used to generate odds ratios (ORs) for evaluating the effect of the intervention for each process indicator for 1, 3, 6, and 12 months postintervention relative to the pre-AAC period (ie, baseline). These analyses account for clustering of visits within practice sites. Process variable performance was also graphically displayed for the intervention sites. Our heterogeneity of treatment effect (HTE) analyses were evaluated by modeling the interaction between the intervention status designated by time period and subgroup (eg, 3 categories of race: Black, White, and other).
Our aim 2 analyses compared adjusted rates for the AAC+TMCP intervention with the AAC-only period (ie, the period between rollout of the AAC intervention and the TMCP intervention using a GEE approach, with robust [jackknife] estimation of variances). Specifically, we modeled the log odds of a particular outcome of interest for a particular patient as a function of intervention status (AAC only vs TMCP+AAC), a random practice site effect, and a random clinician effect in the presence of evidence of substantial clustering at the clinician level within a clinic site. Three analyses were conducted: (1) intention to treat (ITT), (2) per protocol, and (3) per documented use in a visit where the TMCP approach was documented as having been used by completion of the document flow sheet. Outcomes evaluated included referral contact and enrollment rates, provision of tobacco-cessation medications, and quit attempts.
In aim 3 data analysis, we audio-recorded in-depth interviews with 55 primary care patients who agreed to be referred to the QL. These 30-minute interviews included open-ended questions about the patients' experience, with the goal of identifying successes and areas for improvement to address smoking cessation in primary care settings. The interviews, conducted by telephone and audio-recorded, were subsequently transcribed verbatim for analysis. Interviews were based on a semistructured guide designed to explore the experiences of 3 subgroups of patients: those who completed the QL program, those subsequently declining participation in the QL, and those unreachable by the QL. We used a phenomenological approach in our data analysis to understand how people make meaning of their lived experience and to develop a deeper understanding of the common features shared among individuals who agreed to be connected to the QL.17 Analysis began with careful and repeated reading of several transcripts by 3 trained analysts to identify salient themes of the QL referral process (Appendix 5). Based on this initial round of thematic analysis, an initial set of coding categories was created. As additional transcripts were read, the coding categories were modified as necessary to better fit the themes that emerged.
Next, 2 of the analysts each independently coded all 55 transcripts, meeting regularly to discuss coding and reach consensus on any discrepancies. Additionally, the 2 analysts met with a third analyst to review and discuss emerging themes. Interviews were conducted until the point of data saturation was reached for each QL final disposition category (program completed, declined, and unreachable).
Data Management, Quality, and Missing Data
Evaluation of the data for inconsistencies during the analysis phase included checking the range and distribution of all variables and identifying and resolving potential errors. Missing data on patient surveys were negligible (<1%). Principles for maximizing participation and survey completion included keeping the survey short, topic focused, and at a grade 6 reading level or less, with clear and acceptable wording of items and response categories and a clear introduction to the relevance of the measure to the potential participant. EHR fields of interest for this study with information not documented were noted as such in the tables. Missing data for referral outcomes from the QL and FFS were reported. We adopted multiple-imputation strategies to account for the impact of missingness on these outcomes.
Changes to the Original Study Protocol
We extended the intervention follow-up period from 3 months to 6 months for the TMCP phase of the study. Although not an intentional change to the original study design or protocol, 1 of the 8 clinics closed during the study. That clinic received the AAC training but closed shortly after. A replacement clinic was identified from a similar neighborhood, and the original clinic was excluded from all analyses. The replacement clinic received the AAC training and was assigned to receive the TMCP intervention training 1 month after the TMCP training implementation was conducted at the last clinic that had been randomized. This approach allowed sufficient time for data collection and assessment between implementation of the AAC and implementation of the TMCP intervention.
Results
Aim 1
Improve delivery and documentation of tobacco-cessation advice and assistance to socially and economically disadvantaged patients using an AAC approach integrated into the EHR.
During the study period, among the 176 061 patient visits for the pre- and postintervention periods, 26.1% of these patients were identified as smokers. Patient characteristics for the 2 time periods (pre-AAC and post-AAC implementation) are shown in Table 4. There were no substantial differences in any of the patient characteristics (sex, age, race, ethnicity, insurance type, and smoking status) for the 2 periods. Each of the 8 clinics participating in the study contributed a different percentage of patient visits, as expected.
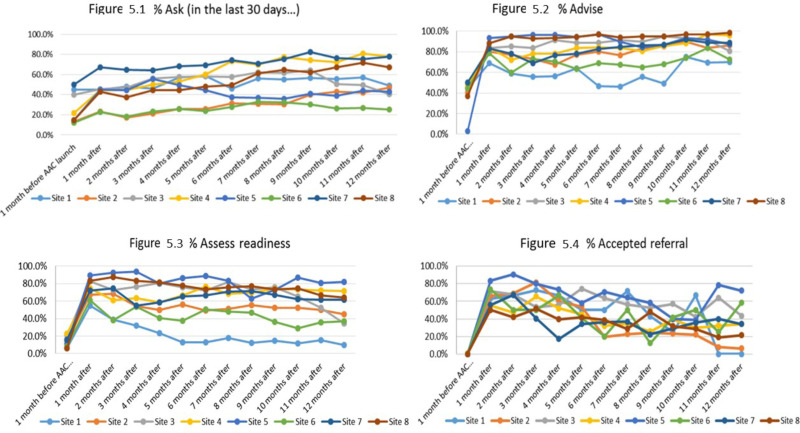
As indicated in Table 5, the pre-AAC implementation period served as the contrast group, and the intervention resulted in a 2-fold increase in asking about smoking status across the 8 sites at the 1-month postintervention time point. This level of impact increased at each subsequent time point and reached OR of 3.7 (95% CI, 3.6-3.9) at 12 months postimplementation. Provision of a brief statement advising patients to quit smoking had a 4-fold increase 1 month after implementation of the AAC intervention. Performance rates increased from 44.8% at baseline to 82.7% at 1 month postimplementation and, with the exception of the 3-month postimplementation time point (78.8%), remained at >80%. For assessing readiness, the baseline rate of 15.8% increased to 74.2% at 1 month after AAC implementation (OR, 14.9; 95% CI, 12.8-17.3). The increase in rates decayed over the 12-month follow-up period to 55% (OR, 6.2; 95% CI, 5.4-7.0). A similar pattern was observed for patient acceptance of an offer for referral to tobacco-cessation counseling (ie, QL or FFS program). The pre-AAC acceptance rate of referrals was 0.5%; after AAC implementation, it was 58.9% (OR, 260.5; 95% CI, 36.9-1840.5). By 12 months after AAC, the rate had decayed to 30.9% but was still dramatically higher than it was during the pre-AAC period (OR, 81.0; 95% CI, 11.4-575.8). Figure 5 shows the effect of the intervention graphically for each of the 8 clinical sites. Six of the sites improved and largely maintained the improvement, while 2 of the sites made marginal improvements, and then declined to baseline levels of performance.
To evaluate the HTE for selected patient visit characteristics, we used a GEE model to examine the pre-AAC and AAC-only periods as well as the intervention effect across subgroups for each process indicator. As shown in Table 6, the percentage of visits for male patients where “Ask” was documented was 24%; this figure increased to 53% after the AAC implementation. For female patients, the percentage was 28%; this figure increased to 50% after the AAC implementation. The sex × time period interaction was significant (χ2 = 80.49; P < .001), which indicates that the intervention had a greater positive impact on documentation of smoking status for visits by male patients vs female patients. For “Ask,” each characteristic examined resulted in a statistically significant interaction effect. One indicator, age, was significant for “Advise.” Given the sample size and thus the power to detect small and clinically nonsignificant differences for “Ask,” we took a conservative approach and interpreted a 10% difference in changes as clinically meaningful. Only 1 variable remained with a significant interaction effect: individuals of “other” race showed a much smaller increase in “Ask” than did White and Black individuals (11.9 vs 23.1 and 27.1, respectively).
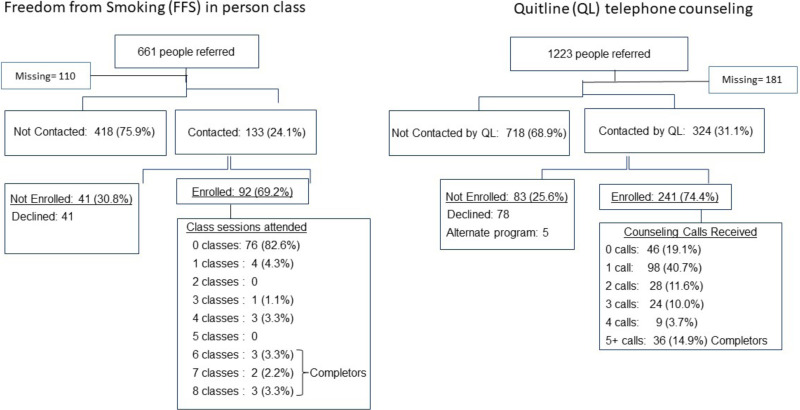
With regard to referrals, among the 661 individuals referred to the FFS program, 551 had outcome data (see Figure 6). A total of 133 (24.1%) were contacted by the program, 92 (69.2%) were enrolled, and 16 (16.3%) attended ≥1 classes. Among patients referred to the QL, 31.1% were contacted by the QL; of those contacted, 74.4% enrolled and 80.7% received ≥1 counseling calls. It is notable that 98 of the 241 patients (40.7%) received only 1 of the recommended 5 counseling calls before unenrolling or becoming unreachable by the QL.
Aim 2
Test the effect of combining TMCP with AAC on process outcomes, QL referral outcomes, and smoking outcomes.
Of the 60 clinicians at the 8 study sites, 44 received the TMCP training (see Table 7). Those who received training were similar in number of years since the last clinical degree, degree type, and internal vs family medicine. Women were significantly underrepresented among those receiving TMCP training (P < .001). Nurse practitioners (NPs) had lower participation, perhaps because they are more likely to work part time and to provide care at ≥1 clinic than physicians, thus decreasing the likelihood of being at the scheduled clinic training. No other characteristics significantly differed between those with TMCP training vs no TMCP training. Among TMCP training participants, 43% were internal medicine and 57% were family medicine specialists; 68% were doctors of medicine, 12% were doctors of osteopathic medicine, and 20% were certified NPs. The average (SD) time since last clinical degree was 16.3 (11) years.
The characteristics of the patient visits across the 3 time periods (ie, pre-AAC, AAC only, and AAC+TMCP) for the overall study are shown in Table 8. There were no substantial differences between the time periods in the characteristics of the patients making visits. Overall, 69% of visits were by female patients, 50% were by White patients, 45% were by Black patients, and 14% were by Hispanic patients. Most had government insurance (ie, Medicaid [40%] or Medicare [24%]); 29% had commercial insurance. About 26% of visits were by individuals who smoked, and 28% were by former smokers. Most (99.9%) patients had their tobacco status documented. The volume of patients seen at each site varied by site but was relatively stable across each time period.
The TMCP training web module was fully completed by all who began it except for 1 clinician, who completed 80%. The average score on the module quiz was 80% correct, and 10 clinicians scored ≥90%. Use of the document flow sheet was used as an indication of uptake of the TMCP approach. Among the 44 clinicians trained, 68% used it ≥1 times during the postintervention period. The median number of uses during the 6 months following the training was 15 (interquartile range, 2-33). Seventeen clinicians (39%) used the document flow sheet >10 times, and 4 used it >50 times. Among the 44 clinicians trained, the proportion of visits by smokers with document flow sheet use ranged from 0% to 21%. In all, a document flow sheet was used in 661 of 8198 (8%) visits by smokers during the evaluation period.
The ITT analyses included all 60 clinicians, regardless of whether they received training; there were no significant increases in any of the process or outcome indicators when we compared the post-AAC period with the post-TMCP period. The per-protocol analyses were examined next. The data in Table 9 are limited to the visits in each time period to 1 of the 8 sites and the 44 clinicians who participated in the TMCP training. As noted in Table 9, there was no meaningful increase in the process variables or patient outcomes from the TMCP training. The AAC intervention was designed to be deployed at every visit, whereas the TMCP intervention was situation specific and driven by identification of and clinician action on a salient concern that arose during the visit. The last column presents the tobacco-cessation support indicators for the visits in which the TMCP document flow sheet was used, indicating that the clinician attempted a teachable moment approach to tobacco cessation. When we examine those visits in which a TMCP approach was documented using the document flow sheet (see the last column of Table 9), we note that when TMCP was documented, “assessing readiness” was performed at a higher rate, and there was a significant increase in the ordering of medications for tobacco cessation (OR, 2.6; 95% CI, 1.9-3.6). No other outcomes improved.
The patient report indicators from the survey were the last outcomes examined across the 3 time periods: pre-AAC, AAC only, and AAC+TMCP. The mean and standard deviation for each patient report indicator for the MA/RN discussion and the discussion by the doctor are reported in Table 10. Across the time periods, patient reports were in the “very good” to “excellent” range (ie, scores of 4-5). Although there were some modest increases in the scores in the AAC-only and AAC+TMCP periods, using analysis of variance and planned paired comparisons evaluated with Tukey tests, none of the differences were statistically significant or clinically meaningful. We also examined the proportion that selected 5 (“excellent”) and compared the 3 time periods and found no meaningful difference in the groups. The scores on the patient report items were high at baseline and remained high in the 2 subsequent intervention periods.
Aim 3
Examine the narratives of subgroups of individuals to better understand the referral experience and identify ways to improve it.
We interviewed 55 participants who accepted a referral by the MA at their primary care clinic. Participants had a median age of 53 years, with the majority being female (75%), Black (58%), and non-Hispanic (93%). Overall, those who participated were similar in age, sex, race, and insurance type to those who did not participate (see Table 11). Data collection was conducted on a rolling basis, and participants were contacted at varying points throughout the e-referral to QL counseling process and in various categories.
Reasons for QL program noncompletion included lack of clarity at the point of referral, changing life circumstances and events making cessation unviable, inconvenient time of phone calls, cell phone difficulties, discomfort with telephone counseling, and having already quit smoking. We also found that some individuals who were no longer receiving QL support–either because they had been classified as program completed, unreachable, or declined – still valued and wanted additional support from the QL. Example quotes to substantiate these themes are included in Table 12.
A common theme among those who had received any QL counseling, including those who at some point disengaged and did not complete the protocol, was that they benefited from the experience. Although some participants reported quitting or cutting down on their smoking, others reported an increased desire to quit, more awareness of their smoking behavior, or some other form of incremental progress in smoking cessation:
It was helpful. It definitely was. When I do wanna smoke a cigarette, that [counseling advice] always plays in my ear. So I'll never forget that every time I pick up a cigarette. So that's making it better for me. [ID 49]
Another finding was that several participants who completed the QL program or were no longer receiving calls because they had been categorized as having declined or being unreachable, desired continued cessation support. Some specifically wanted to re-engage with the QL:
Yes, I would [like to talk to the QL], because it was just a misunderstanding and the wrong moment. That's all it was. [ID 9]
Yeah. I'd still like to talk to them. That way we can still set goals and have the motivation to keep going. [ID 54]
With regard to the larger, primary care context of smoking cessation, the overwhelming majority of patients reported wanting providers' continued engagement and support with smoking cessation. When asked what role they wanted their primary care providers to play in their smoking cessation, participants expressed the desire for ongoing assistance and encouragement:
“If this don't work, let's go to the next level,” you know. In other words, let's not give up on me. You know, “Come on—you keep trying until we find the right thing for you.” That's what I want. [ID 14]
Just check up to see how I'm doing. Am I still smoking? Am I not smoking? “How are you doing with your smoking?” or “If you are still smoking, is there something else that we can do to help you stop smoking?” Those type of questions. [ID 42]
Discussion
The implementation of the AAC strategy resulted in a large, statistically significant increase in performance and documentation of tobacco-cessation assistance. Although the uptake of all the new tasks was good, the most robust uptake and sustained performance was associated with the provision of brief advice. This finding may be a result of 2 factors. First is the health care system's preference for implementing a routinized approach to addressing tobacco assessment and cessation assistance for every primary care patient at every visit. Second, the study team extensively engaged MAs as representatives of the frontline users of the approach during the development and implementation phase. The MAs were instrumental in developing the wording of the brief advice phrase, which resulted in a phrase that the MAs were comfortable using. In addition, we anticipated that the overall approach would be empowering to the MAs because the AAC strategy enables them to sign the referral order for external tobacco-cessation counseling. Overall, we found that the successful and sustained adoption of AAC was in large part the result of its being embedded within and supported by the health care system and designed and implemented in partnership with the end users.42 Greenwood et al43 also found that MA role expansion and empowerment to support tobacco-cessation care improved documentation of smoking status and referrals to telephone-based cessation counseling (ie, QL) significantly. Training various clinical support staff to assess tobacco status, provide brief advice, and assist or refer patients for assistance is reported as efficacious in a variety of health care settings in a growing number of studies,44-46 although none to our knowledge has measured the sustained effect at 12 months postimplementation. Shifting some of the responsibility for tobacco-cessation support to nonphysicians can be an effective strategy, particularly as a component of larger system change.
Our approach to training and technical assistance for implementation of the AAC strategy had good fidelity across each of the 8 community health centers. However, there was substantial variation in the uptake of assessing readiness and therefore offering referral to the QL for the 8 sites. Several similar studies have documented barriers that can contribute to variability in uptake across practices and individual staff members. Possible explanations offered include high staff turnover combined with no systematic process for training new staff on the protocol,45 hesitation or discomfort with asking every patient at every visit about smoking,43 and varying levels of EHR competency.45
It is encouraging that there was minimal HTE of the AAC intervention; this effect was limited to a few visit characteristics for the “Ask” process indicator. Although the performance of the AAC process was sustained at most practices for most indicators for 12 months, patient acceptance of assistance to quit smoking decreased after 6 months. It is likely that individuals who had return visits and who encountered the AAC process at each subsequent visit may have already engaged once but either continued to use tobacco or, as indicated from the qualitative findings, may have already quit.
This is the first study to engage clinicians in the TMCP approach as part of a systems-change intervention. Prior work engaged clinicians as study volunteers.34,35 The training was conducted at the practice site during a clinical work day and used protected administrative time granted by the health care system. Feedback solicited from the clinicians about the web module, the skill practice section, and the place and timing of the training was positive. The TMCP approach and training were viewed as both important and pragmatic. This study also developed and deployed a document flow sheet, which is a common EHR tool, to both cue and document the TMCP. During the training, coaches observed substantial variability in clinician willingness to use the document flow sheet while learning the new communication process. The overall uptake of the TMCP approach in routine patient care was poor; a document flow sheet indicated use of the TMCP approach in 8% of visits by tobacco users. This low rate of uptake limits the ability to evaluate the impact of its use on patient outcomes. Variation in clinician uptake of tobacco-cessation counseling training has been reported as resulting from variation in clinicians' beliefs regarding the importance of cessation.47,48 Like other EHR tool implementation efforts, our findings show significant variability in the adoption and use of this EHR tool despite specific training and technical assistance.45 Unlike the AAC approach, which was built into existing work flows, the TMCP was designed to be used at the clinician's discretion when a salient patient concern made discussion of tobacco cessation relevant to the visit. Further, a technical limitation of the implementation of the TMCP approach was that uniting the AAC activity (ie, patient ready to quit declined referral to tobacco-cessation services) with the clinician dashboard view of the EHR was not sufficiently achieved. Thus, there was no way to prompt clinicians about potentially activated patients. Nonetheless, when the TMCP approach was used, it substantially increased the orders for tobacco-cessation medications compared with the AAC-only period. This is important in that tobacco-cessation counseling and medications to support a quit attempt together increase the likelihood of a quit attempt.49,50 To realize the potential of the TMCP approach to augment patient support and the effectiveness of tobacco-cessation attempts, systems change work is needed to better integrate and align the AAC activity with the EHR view for clinicians. Doing this in a way that is technically feasible and acceptable to clinicians by fitting it into the workflow is essential. Clinician EHR alert fatigue is a concern, and continued engagement of frontline clinicians and clinician informatics experts is essential to meaningfully designing an EHR interface that supports the care-delivery process in a nondisruptive way. Other strategies that could be considered in future research to improve clinician uptake include conducting audits and gathering feedback on performance51 as well as providing booster training.
An important aspect of this study was examining the tobacco-cessation support process from the perspective of patients. We elicited the experiences from QL-referred smokers to understand the overall experience and to identify reasons for and circumstances surrounding their noncompletion of a state QL's 5-session counseling protocol. By interviewing not only participants who completed the program but also those who at some point declined to be involved or were unreachable by the QL, we have expanded understanding of why rates of noncompletion are high. We found that a major reason for noncompletion was having significant life stressors, such as poor living conditions and long and/or unpredictable work hours. Others have also found that stressors related to poor socioeconomic status are a barrier to use of QL counseling.52,53 Integrating QL interventions with community-based resources that address the sociocontextual mediators of tobacco use could be a promising strategy.54 Discomfort with telephone-based counseling was found to be another reason for QL disengagement. Some researchers have improved QL engagement by incorporating culturally specific adaptations55,56 because different ethnic/racial groups can have differing norms and values with regard to smoking and experience unique barriers to cessation.57,58 The potential for QLs to reach and support the population of smokers is significant, but addressing some of these barriers in future research is warranted. Finding ways to increase engagement in tobacco-cessation counseling support is important for improving the likelihood of a successful quit attempt. Piñeiro et al found that those who completed ≥3 sessions had the greatest likelihood of achieving abstinence and were approximately 4 times as likely to be abstinent at 6 months compared with those who completed no sessions or only 1 session.59
The categories “program complete” and “quit status” are often used by QLs as indicators of the degree of QL engagement and the success of a tobacco-cessation program. Our findings suggest that these categories do not tell the whole story. Many participants who did not complete the program found great value in their engagement with the QL and accomplished goals such as quitting or cutting down on their use of tobacco products. Because smoking cessation is a process that frequently involves several quit attempts as well as a behavior change that needs to be sustained over time, the QL can potentially play an important role regardless of where a patient may be along the tobacco-cessation trajectory. We found that some individuals who were no longer receiving QL support, because they had been classified as program completed, unreachable, or declined, expressed that they would value ongoing or future support from the QL. Other studies have found that many relapsed smokers are interested in re-entering treatment60,61 and that interventions to encourage past QL participants to return to services and reinitiate QL-assisted quit attempts are effective.62,63
Finally, we found that study participants want ongoing communication with their primary care providers about smoking and smoking cessation. Regardless of their quit status, patients appreciate their providers checking in with them, offering encouragement, and working with them to solve problems with cessation strategies. Closed-loop EHR referral systems that include delivery of treatment information from the QL back to the primary care team may help facilitate ongoing patient-provider communication.25
Subpopulation Considerations
This study was conducted in a setting with a high proportion of socially and economically disadvantaged patients. Those with low income and lower levels of education are more likely to use tobacco and thus are an important group to reach with interventions designed to improve tobacco-cessation support in the context of the primary care setting. As noted previously, the AAC intervention appeared to work equally well for all subgroups.
Study Limitations
The study sample was drawn from a health care system (MetroHealth) that serves patients who tend to have very low income. Thus, socioeconomic barriers to engaging with the QL were prevalent. The smoking rate in the primary care practices at this system is about 30%; the approach to a routinized, “every patient, every visit” strategy may be less efficient where smoking rates are lower. The calculation of a quit attempt assumes documentation of a quit date in the EHR. However, in some instances, a quit attempt may have been made by a patient without supportive documentation in their record. Consequently, our quit attempt estimate serves as a lower bound for the actual quit attempt rate. Although we observed variation in uptake across the 8 clinical sites of the AAC process, data collection did not include practice-level variables that may explain some of this variation. Finally, uptake of the TMCP approach by clinicians (as recorded by the document flow sheet) was poor and severely limited our ability to evaluate the impact of the TMCP approach. Low TMCP use across most clinicians and the small number of TMCP uses overall resulted in poor statistical power to evaluate the effect of the TMCP approach on outcomes. Despite these limitations, the findings and the utility of the intervention components tested in this study (ie, using EHR e-referral to a state QL, routine training methods supported by audit and feedback on performance) are relevant and applicable to other primary care settings.
Conclusions
The AAC system change intervention substantially increased the provision of tobacco-cessation care, with improvements sustained beyond 1 year. Adding TMCP training for clinicians improved ordering of tobacco cessation medications, but other outcomes did not improve. However, low uptake limited our ability to test the impact of the TMCP approach on patient outcomes. This pragmatic cluster RCT indicated the value of a system-based approach and the importance of structures that fully support the integration of simultaneously implemented interventions by the primary health care team. Future work will require more complete integration of the AAC and TMCP approaches and tools into the EHR systems for the combined process to be fully tested.
References
- 1.
- Cokkinides VE, Halpern MT, Barbeau EM, Ward E, Thun MJ. Racial and ethnic disparities in smoking-cessation interventions: analysis of the 2005 National Health Interview Survey. Am J Prev Med. 2008;34(5):404-412. [PubMed: 18407007]
- 2.
- Fix BV, Hyland A, Rivard C, et al. Usage patterns of stop smoking medications in Australia, Canada, the United Kingdom, and the United States: findings from the 2006-2008 International Tobacco Control (ITC) Four Country Survey. Int J Environ Res Public Health. 2011;8(1):222-233. [PMC free article: PMC3037071] [PubMed: 21318025]
- 3.
- Fu SS, Sherman SE, Yano EM, van Ryn M, Lanto AB, Joseph AM. Ethnic disparities in the use of nicotine replacement therapy for smoking cessation in an equal access health care system. Am J Health Promot. 2005;20(2):108-116. [PubMed: 16295702]
- 4.
- Trinidad DR, Perez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health. 2011;101(4):699-706. [PMC free article: PMC3052356] [PubMed: 21330593]
- 5.
- US Department of Health and Human Services. Quitting smoking among adults—United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60(44):1513-1519. [PubMed: 22071589]
- 6.
- Centers for Disease Control and Prevention/National Center for Health Statistics. National Health Interview Survey, 2011 Data Release. Accessed February 3, 2021. https://www
.cdc.gov/nchs /nhis/nhis_2011_data_release.htm - 7.
- Thorndike AN, Regan S, Rigotti NA. The treatment of smoking by US physicians during ambulatory visits: 1994 2003. Am J Public Health. 2007;97(10):1878-1883. [PMC free article: PMC1994176] [PubMed: 17761570]
- 8.
- National Prevention Council. National Prevention Strategy. Published June 2011. Accessed August 8, 2020. https://www
.hhs.gov/sites /default/files/disease-prevention-wellness-report.pdf - 9.
- Tobacco Use and Dependence Guideline Panel. Treating Tobacco Use and Dependence: 2008 Update. US Department of Health and Human Services; 2008. Accessed February 3, 2021. https://www
.ncbi.nlm .nih.gov/books/NBK63952/ - 10.
- North American Quitline Consortium. Quality Improvement Initiative Issue Paper: Quitline Referral Systems. NAQC Issue Paper. 2013. Accessed August 8, 2020. https://cdn
.ymaws.com/sites/naquitline .site-ym .com/resource/resmgr /Issue_Papers/QuitlineReferralSystemsQuali.pdf - 11.
- Ossip-Klein DJ, McIntosh S. Quitlines in North America: evidence base and applications. Am J Med Sci. 2003;326(4):201-205. [PubMed: 14557735]
- 12.
- Matkin W, Ordóñez-Mena JM, Hartmann-Boyce J. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2019 May 2;5(5):CD002850. doi:10.1002/14651858.CD002850.pub4 [PMC free article: PMC6496404] [PubMed: 31045250] [CrossRef]
- 13.
- Fiore MC. Treating tobacco use and dependence: an introduction to the US Public Health Service Clinical Practice Guideline. Respir Care. 2000;45(10):1196-1199. [PubMed: 11203101]
- 14.
- Riley WT, Glasgow RE, Etheredge L, Abernethy AP. Rapid, responsive, relevant (R3) research: a call for a rapid learning health research enterprise. Clin Transl Med. 2013;2(1):10. [PMC free article: PMC3658895] [PubMed: 23663660]
- 15.
- Simons VA, Flynn SP, Flocke SA. Practical behavior change counseling in primary care. Prim Care. 2007;34(3):611-622, vii. [PubMed: 17868762]
- 16.
- US Preventive Services Task Force. The Guide to Clinical Preventive Services 2010-2011: Recommendations of the US Preventive Services Task Force. Report No. 10-05145. Agency for Healthcare Research and Quality; 2010. Accessed August 8, 2020. https://www
.ncbi.nlm .nih.gov/books/NBK56707 [PubMed: 21850778] - 17.
- Wasserman MP. Guide to community preventive services: state and local opportunities for tobacco use reduction. Am J Prev Med. 2001;20(2 Suppl):8-9. [PubMed: 11173213]
- 18.
- North American Quitline Consortium. The Use of Quitlines Among Priority Populations in the U.S.: Lessons From the Scientific Evidence. NAQC Issue Paper. 2011. Accessed August 8, 2020. https://cdn
.ymaws.com/www .naquitline.org /resource/resmgr/Issue_Papers /IssuePaperTheUseofQuitlinesA .pdf - 19.
- Bentz CJ, Bayley KB, Bonin KE, et al. Provider feedback to improve 5A's tobacco cessation in primary care: a cluster randomized clinical trial. Nicotine Tob Res. 2007;9(3):341-349. [PubMed: 17365766]
- 20.
- Redmond LA, Adsit R, Kobinsky KH, Theobald W, Fiore MC. A decade of experience promoting the clinical treatment of tobacco dependence in Wisconsin. WMJ. 2010;109(2):71-78. [PubMed: 20443325]
- 21.
- Rothemich SF, Woolf SH, Johnson RE, et al. Promoting primary care smoking-cessation support with quitlines: the QuitLink Randomized Controlled Trial. Am J Prev Med. 2010;38(4):367-374. [PubMed: 20307804]
- 22.
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;(5):CD000165. doi:10.1002/14651858.CD000165.pub4 [PMC free article: PMC7064045] [PubMed: 23728631] [CrossRef]
- 23.
- Warner DD, Land TG, Rodgers AB, Keithly L. Integrating tobacco cessation quitlines into health care: Massachusetts, 2002-2011. Prev Chronic Dis. 2012;9:110343. doi:10.5888/pcd9.110343 [PMC free article: PMC3468303] [PubMed: 22840885] [CrossRef]
- 24.
- Mathew M, Goldstein AO, Kramer KD, Ripley-Moffitt C, Mage C. Evaluation of a direct mailing campaign to increase physician awareness and utilization of a quitline fax referral service. J Health Commun. 2010;15(8):840-845. [PubMed: 21170786]
- 25.
- Adsit RT, Fox BM, Tsiolis T, et al. Using the electronic health record to connect primary care patients to evidence-based telephonic tobacco quitline services: a closed-loop demonstration project. Transl Behav Med. 2014;4(3):324-332. [PMC free article: PMC4167898] [PubMed: 25264471]
- 26.
- Cohen DJ, Clark EC, Lawson PJ, Casucci BA, Flocke SA. Identifying teachable moments for health behavior counseling in primary care. Patient Educ Couns. 2011;85(2):e8-e15. doi:10.1016/j.pec.2010.11.009 [PMC free article: PMC4389220] [PubMed: 21183305] [CrossRef]
- 27.
- Flocke SA, Clark E, Antognoli E, et al. Teachable moments for health behavior change and intermediate patient outcomes. Patient Educ Couns. 2014;96(1):43-49. [PMC free article: PMC4427843] [PubMed: 24856449]
- 28.
- Flocke SA, Stange KC. Direct observation and patient recall of health behavior advice. Prev Med. 2004;38(3):343-349. [PubMed: 14766118]
- 29.
- Lawson PJ, Flocke SA. Teachable moments for health behavior change: a concept analysis. Patient Educ Couns. 2009;76(1):25-30. [PMC free article: PMC2733160] [PubMed: 19110395]
- 30.
- Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173(6):458-464. [PMC free article: PMC3858085] [PubMed: 23440173]
- 31.
- Vidrine JI, Shete S, Li Y, et al. The Ask-Advise-Connect approach for smokers in a safety net healthcare system: a group-randomized trial. Am J Prev Med. 2013;45(6):737-741. [PMC free article: PMC4023543] [PubMed: 24237916]
- 32.
- Sadasivam RS, Hogan TP, Volkman JE, et al. Implementing point of care “e-referrals” in 137 clinics to increase access to a quit smoking internet system: the Quit-Primo and National Dental PBRN HI-QUIT studies. Transl Behav Med. 2013;3(4):370-378. [PMC free article: PMC3830021] [PubMed: 24294325]
- 33.
- Willett JG, Hood NE, Burns EK, et al. Clinical faxed referrals to a tobacco quitline: reach, enrollment, and participant characteristics. Am J Prev Med. 2009;36(4):337-340. [PubMed: 19201150]
- 34.
- Flocke SA, Antognoli E, Step MM, Marsh S, Parran T, Mason MJ. A Teachable Moment Communication Process for smoking cessation talk: description of a group randomized clinician-focused intervention. BMC Health Serv Res. 2012;12:109. doi:10.1186/1472-6963-12-109 [PMC free article: PMC3529679] [PubMed: 22554310] [CrossRef]
- 35.
- Flocke SA, Step MM, Antognoli E, et al. A randomized trial to evaluate primary care clinician training to use the Teachable Moment Communication Process for smoking cessation counseling. Prev Med. 2014;69:267-273. [PMC free article: PMC4312229] [PubMed: 25456811]
- 36.
- Weiner BJ, Lewis MA, Clauser SB, Stitzenberg KB. In search of synergy: strategies for combining interventions at multiple levels. J Natl Cancer Inst Monogr. 2012;2012(44):34-41. [PMC free article: PMC3482963] [PubMed: 22623594]
- 37.
- Adams B, Allan T, Cheatham C, et al. 2018 Cuyahoga County Community Health Assessment: A Community Health Needs Assessment. 2018. Accessed August 8, 2020. https://hipcuyahoga.org/wp-content/uploads/2018/11/2018CuyahogaCountyAssessmentFinal.pdf
- 38.
- Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28(2):182-191. [PubMed: 16829207]
- 39.
- Lawson PJ, Flocke SA, Casucci B. Development of an instrument to document the 5A's for smoking cessation. Am J Prev Med. 2009;37(3):248-254. [PMC free article: PMC2735336] [PubMed: 19666161]
- 40.
- Makoul G, Krupat E, Chang CH. Measuring patient views of physician communication skills: development and testing of the Communication Assessment Tool. Patient Educ Couns. 2007;67(3):333-342. [PubMed: 17574367]
- 41.
- Halladay JR, Vu M, Ripley-Moffitt C, Gupta SK, O'Meara C, Goldstein AO. Patient perspectives on tobacco use treatment in primary care. Prev Chronic Dis. 2015;12:140408. doi:10.5888/pcd12.140408 [PMC free article: PMC4318687] [PubMed: 25654219] [CrossRef]
- 42.
- Flocke SA, Seeholzer E, Lewis SA, et al. Designing for sustainability: an approach to integrating staff role changes and electronic health record functionality within safety-net clinics to address provision of tobacco cessation care. Jt Comm J Qual Patient Saf. 2019;45(21):798-807. [PubMed: 31648946]
- 43.
- Greenwood DA, Parise CA, MacAller TA, et al. Utilizing clinical support staff and electronic health records to increase tobacco use documentation and referrals to a state quitline. J Vasc Nurs. 2012;30(4):107-111. [PubMed: 23127426]
- 44.
- Chavarria J, Liu M, Kast L, Salem E, King AC. A pilot study of Counsel to Quit: evaluating an Ask Advise Refer (AAR)-based tobacco cessation training for medical and mental healthcare providers. J Subst Abuse Treat. 2019;99:163-170. [PubMed: 30797390]
- 45.
- Karn S, Fernandez A, Grossberg LA, et al. Systematically improving tobacco cessation patient services through electronic medical record integration. Health Promot Pract. 2016;17(4):482-489. [PubMed: 27199147]
- 46.
- Wye PM, Stockings EA, Bowman JA, Oldmeadow C, Wiggers JH. Effectiveness of a clinical practice change intervention in increasing the provision of nicotine dependence treatment in inpatient psychiatric facilities: an implementation trial. BMC Psychiatry. 2017;17(1):56. doi:10.1186/s12888-017-1220-7 [PMC free article: PMC5297214] [PubMed: 28173823] [CrossRef]
- 47.
- Papadakis S, Cole AG, Reid RD, et al. From good to great: the role of performance coaching in enhancing tobacco-dependence treatment rates. Ann Fam Med. 2018;16(6):498-506. [PMC free article: PMC6231943] [PubMed: 30420364]
- 48.
- Papadakis S, Cole AG, Reid RD, et al. Increasing rates of tobacco treatment delivery in primary care practice: evaluation of the Ottawa Model for Smoking Cessation. Ann Fam Med. 2016;14(3):235-243. [PMC free article: PMC4868562] [PubMed: 27184994]
- 49.
- Silfen SL, Cha J, Wang JJ, Land TG, Shih SC. Patient characteristics associated with smoking cessation interventions and quit attempt rates across 10 community health centers with electronic health records. Am J Public Health. 2015;105(10):2143-2149. [PMC free article: PMC4566564] [PubMed: 25880939]
- 50.
- Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;(3):CD008286. doi:10.1002/14651858.CD008286.pub3 [PMC free article: PMC10042551] [PubMed: 27009521] [CrossRef]
- 51.
- Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. doi:10.1002/14651858.CD000259.pub3 [PubMed: 22696318] [CrossRef]
- 52.
- Sheffer CE, Brackman SL, Cottoms N, Olsen M. Understanding the barriers to use of free, proactive telephone counseling for tobacco dependence. Qual Health Res. 2011;21(8):1075-1085. [PMC free article: PMC3369823] [PubMed: 21464470]
- 53.
- Varghese M, Sheffer C, Stitzer M, Landes R, Brackman SL, Munn T. Socioeconomic disparities in telephone-based treatment of tobacco dependence. Am J Public Health. 2014;104(8):e76-e84. doi:10.2105/AJPH.2014.301951 [PMC free article: PMC4103213] [PubMed: 24922165] [CrossRef]
- 54.
- Haas JS, Linder JA, Park ER, et al. Proactive tobacco cessation outreach to smokers of low socioeconomic status: a randomized clinical trial. JAMA Intern Med. 2015;175(2):218-226. [PMC free article: PMC4590783] [PubMed: 25506771]
- 55.
- Butler KM, Begley K, Riker C, et al. Smoke-free coalition cohesiveness in rural tobacco-growing communities. J Community Health. 2014;39(3):592-598. [PMC free article: PMC4004689] [PubMed: 24338076]
- 56.
- Webb Hooper M, Carpenter K, Salmon EE. Web-based tobacco cessation interventions and digital inequality across U.S. racial/ethnic groups. Ethn Dis. 2019;29(3):495-504. [PMC free article: PMC6645725] [PubMed: 31367170]
- 57.
- Daoud N, Jung YE, Sheikh Muhammad A, et al. Facilitators and barriers to smoking cessation among minority men using the behavioral-ecological model and Behavior Change Wheel: a concept mapping study. PLoS One. 2018;13(10):e0204657. doi:10.1317/journal.pone.0204657 [PMC free article: PMC6200188] [PubMed: 30356254] [CrossRef]
- 58.
- Nierkens V, Hartman MA, Nicolaou M, et al. Effectiveness of cultural adaptations of interventions aimed at smoking cessation, diet, and/or physical activity in ethnic minorities. a systematic review. PLoS One. 2013;8(10):e73373. doi:10.1371/journal.pone.0073373 [PMC free article: PMC3792111] [PubMed: 24116000] [CrossRef]
- 59.
- Piñeiro B, Wetter DW, Vidrine DJ, et al. Quitline treatment dose predicts cessation outcomes among safety net patients linked with treatment via Ask-Advise-Connect. Prev Med Rep. 2019;13:262-267. [PMC free article: PMC6351387] [PubMed: 30723660]
- 60.
- Fu SS, Partin MR, Snyder A, et al. Promoting repeat tobacco dependence treatment: are relapsed smokers interested? Am J Manag Care. 2006;12(4):235-243. [PubMed: 16610925]
- 61.
- Partin MR, An LC, Nelson DB, et al. Randomized trial of an intervention to facilitate recycling for relapsed smokers. Am J Prev Med. 2006;31(4):293-299. [PubMed: 16979453]
- 62.
- Carlini B, Miles L, Doyle S, Celestino P, Koutsky J. Using diverse communication strategies to re-engage relapsed tobacco quitline users in treatment, New York State, 2014. Prev Chronic Dis. 2015;12:E179. doi:10.5888/pcd12.150191 [PMC free article: PMC4617459] [PubMed: 26491814] [CrossRef]
- 63.
- Carlini BH, McDaniel AM, Weaver MT, et al. Reaching out, inviting back: using interactive voice response (IVR) technology to recycle relapsed smokers back to quitline treatment—a randomized controlled trial. BMC Public Health. 2012;12:507. doi:10.1186/1471-2458-12-507 [PMC free article: PMC3438078] [PubMed: 22768793] [CrossRef]
Related Publications
- Flocke SA, Lewis S, Seeholzer E, et al. Electronic medical record documentation of tobacco cessation support at eight community safety-net clinics with a high prevalence of tobacco use. J Eval Clin Pract. 2019;25(3):507-513. [PubMed: 30456776]
- Flocke SA, Seeholzer E, Lewis S, et al. Designing for sustainability: an approach to integrating staff role changes and electronic health record functionality within safety-net clinics to address provision of tobacco cessation care. Jt Comm J Qual Patient Saf. 2019;45(12):798-807. [PubMed: 31648946]
- Flocke SA, Seeholzer E, Lewis SA, Gill IJ, Rose JC, Albert EL, Love TE, Kaelber D. 12 month evaluation of an EHR-supported staff role change for provision of tobacco cessation care in 8 primary care safety-net clinics. J Gen Intern Med. 2020 Nov;35(11):3234-3242. [PMC free article: PMC7661631] [PubMed: 32705473]
- Albert EL, Rose JC, Gill IJ, Flocke SA. Quitting the quitline: a qualitative study of patient experience of electronic referrals to quitlines. BMC Public Health. 2020 Jul 9;20(1):1080. [PMC free article: PMC7350715] [PubMed: 32646397]
Acknowledgments
This research was funded through PCORI Award IHS-1503-29879. The statements in this report are solely the responsibility of the authors and do not necessarily represent the views of PCORI, its Board of Governors, or its Methodology Committee. We acknowledge the support of all our project partners, including MetroHealth System, the Ohio Department of Health, and the Ohio Quitline provider National Jewish Health. We thank all MetroHealth care providers, staff, and patients who made this research possible. We wish to thank Georgene Bosich, RN; Jay Koren, RN; Versie Owens, MPA; and Teodoro Rosati for their outstanding contributions to this project.
Research reported in this report was funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (#IHS-1503-29879-IC). Further information available at: https://www.pcori.org/research-results/2015/adding-teachable-moment-approach-team-based-primary-care-ask-advise-connect
Appendices
Appendix 1.
AAC Process Description (PDF, 262K)
Appendix 2.
TMCP Description (PDF, 161K)
Appendix 3.
Participant Consent Process (PDF, 97K)
Appendix 4.
Tobacco Cessation Patient Survey (PDF, 107K)
Appendix 5.
In-depth Interview Approach (PDF, 91K)
Figures
Figure 3AAC and TMCP Intervention Implementation Dates and Times Before, Between, and After Interventions for Each Site
Abbreviations: AAC, Ask-Advise-Connect; TMCP, Teachable Moment Communication Process.
*Because of technical problems for some clinicians, a second TMCP implementation was conducted on November 2.
**This site replaced one that had been randomized but that closed shortly after receiving AAC training.
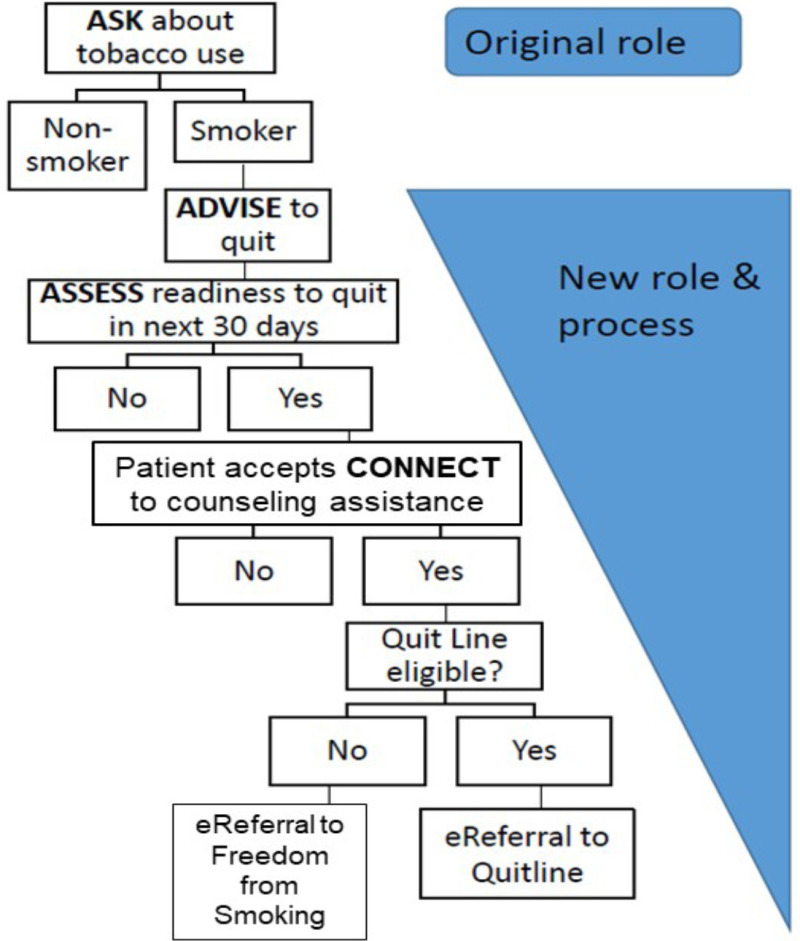
Figure 4MA/RN Roles and Process Changes With AAC System Intervention
Abbreviations: AAC, Ask-Advise-Connect; MA, medical assistant; RN, registered nurse.
Tables
Table 1Interventions, Influences, and Outputs
| Intervention features | Influence | Reach | Outputs | |
|---|---|---|---|---|
| AAC |
|
Increase structural capacity to do the following:
|
|
|
| TMCP |
|
|
|
|
Abbreviations: AAC, Ask-Advise-Connect; EHR, electronic health record; MA, medical assistant; QL, quitline; RN, registered nurse; TMCP, Teachable Moment Communication Process.
Table 2AAC Components, EHR Functionality Changes, and Role Expectations
| Component | EHR button | Guiding phrases in EHR | Role expectation before AAC implementation | Role expectation after AAC implementation |
|---|---|---|---|---|
| Ask | Existing | Existing: Have you used a tobacco product in the past 30 days? | MA | MA |
| Advise | Existing | NEW: As a member of your health care team, we strongly recommend that you quit using tobacco. | Clinician | MA |
| Assess readiness | Existing | NEW: Do you want to quit in the next 30 days? | Clinician | MA |
| Assess interest in referral | NEW | NEW: Would you like us to connect you to a coach who can help you quit? | Clinician | MA |
| Connect (ie, order referral) | Existing but only for in-house program NEW for QL | NEW: I will place an order for you and someone will call you in the next day or two. | Clinician | MA |
Abbreviations: AAC, Ask-Advise-Connect; EHR, electronic health record; MA, medical assistant; QL, quitline.
Table 3Study Outcomes and Data Sources
| Data source: EHR | Population |
|---|---|
| Assessment of the degree to which these functions are happening: | All visits by patients aged ≥18 y to 1 of the 8 clinics during the study period |
| Ask: smoking status ascertained | |
| Advise: recommended quitting | |
| Assess: assessed readiness to quit now | |
| Connect: offered referral to assistance with quitting (ie, QL or FFS services) | |
| Patient's smoking status | |
| Current smoker, previous smoker, nonsmoker | |
| Patient's readiness to quit in the next 30 d | |
| Referral accepted or declined | |
| Data source: referral data returned to EHR | |
| QL | Patients who accepted a referral to tobacco-cessation counseling |
| Successfully contacted | |
| Enrolled | |
| No. of counseling sessions completed | |
| FFS | |
| Successfully contacted | |
| Enrolled | |
| No. of counseling sessions completed | |
| Received NRT | |
| Data source: patient survey | Population |
| Items measuring patient experience with the primary care teama | Random samples of patient aged ≥18 y who smoked were seen during the study period and had a discussion about smoking |
| Example items: | |
| Were you treated with respect? | |
| Did you feel listened to? | |
| Were you able to honestly speak your mind about quitting? | |
| Was the advice you received helpful? | |
| Data source: in-depth interviews | |
| Patient narratives about experiences with e-referral to the QL | 3 groups from the sample of patients who agreed to be referred to the QL and subsequently (1) declined participation in the QL, (2) were unreachable by the QL, (3) completed the QL program |
| Key covariates | |
| EHR | |
| Clinic site | |
| Provider type: family medicine, internal medicine | |
| Patient's primary insurance: commercial, Medicare, Medicaid, uninsured | |
| Patient demographics: age, sex, race/ethnicity |
Abbreviations: EHR, electronic health record; FFS, Freedom From Smoking; NRT, nicotine replacement therapy; QL, quitline.
aItem selection informed by patient stakeholders. Items rated on a 5-point scale.
Table 4Visit Characteristics for Pre-AAC and Post–AAC-Only Implementation
| Description | Overall (N = 176 061 [100.0%]), No. (%) | Pre-AAC implementation (n = 36 677 [20.8%]), No. (%) | Post–AAC-only implementation (n = 139 384 [79.2%]), No. (%) |
|---|---|---|---|
| Sex | |||
| Male | 53 518 (30.4) | 11 338 (30.9) | 42 180 (30.3) |
| Female | 122 542 (69.6) | 25 338 (69.1) | 97 204 (69.7) |
| Age, y | |||
| 18-34 | 43 804 (24.9) | 9099 (24.8) | 34 705 (24.9) |
| 35-64 | 100 851 (57.3) | 21 400 (58.3) | 79 451 (57.0) |
| ≥65 | 31 406 (17.8) | 6178 (16.8) | 25 228 (18.1) |
| Race | |||
| White | 79 138 (50.0) | 15 867 (48.0) | 63 271 (50.5) |
| Black | 73 320 (46.3) | 16 048 (48.6) | 57 272 (45.7) |
| Other | 5802 (3.7) | 1108 (3.4) | 4694 (3.7) |
| Ethnicity | |||
| Non-Hispanic | 150 782 (87.9) | 31 531 (88.2) | 119 251 (87.8) |
| Hispanic | 20 840 (12.1) | 4209 (11.8) | 16 631 (12.2) |
| Primary insurance class | |||
| Commercial | 51 484 (29.9) | 10 470 (29.5) | 41 014 (30.1) |
| Medicaid | 68 714 (40.0) | 14 736 (41.5) | 53 978 (39.6) |
| Medicare | 41 014 (23.9) | 8285 (23.3) | 32 729 (24.0) |
| Self-pay | 10 540 (6.1) | 1979 (5.6) | 8561 (6.3) |
| Other | 206 (0.1) | 34 (0.1) | 172 (0.1) |
| Smoking status | |||
| Current smoker | 37 909 (26.1) | 8167 (26.7) | 29 742 (25.9) |
| Former smoker | 40 732 (28.0) | 8525 (27.8) | 32 207 (28.0) |
| Never smoked | 66 873 (45.9) | 13 953 (45.5) | 52 920 (44.3) |
| Not assesseda | 30 547 (17.4) | 6032 (16.4) | 24 515 (17.6) |
| MetroHealth center | |||
| A | 14 299 (8.1) | 3003 (8.2) | 11 296 (8.1) |
| B | 31 704 (18.0) | 5899 (16.1) | 25 805 (18.5) |
| C | 22 053 (12.5) | 3727 (10.2) | 18 326 (13.1) |
| D | 21 348 (12.1) | 4521 (12.3) | 16 827 (12.1) |
| E | 5434 (3.1) | 2573 (7.0) | 2861 (2.1) |
| F | 21 757 (12.4) | 4680 (12.8) | 17 077 (12.3) |
| G | 33 526 (19.0) | 6862 (18.7) | 26 664 (19.1) |
| H | 20 587 (11.7) | 4399 (12.0) | 16 188 (11.6) |
| I | 5353 (3.0) | 1013 (2.8) | 4340 (3.1) |
Abbreviation: AAC, Ask-Advise-Connect.
aNot included in the denominator for smoking status.
Table 5Process Variables for the 3 Months Before Implementing AAC and Months 1, 3, 6, and 12 After Implementing AAC
| 1-3 mo before AAC (Baseline) | 1 mo after starting AACa | 3 mo after starting AAC | 6 mo after starting AAC | 12 mo after starting AAC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | OR (95% CI) | No. | % | OR (95% CI) | No. | % | OR (95% CI) | No. | % | OR (95% CI) | No. | % | OR (95% CI) | |
| Total visits | 36 677 | 12 274 | 12 012 | 11 605 | 11 991 | ||||||||||
| % of patients asked about smoking | 9742 | 26.6 | – | 5035 | 41.0 | 1.93 (1.85-2.00) | 5437 | 45.3 | 2.33 (2.24-2.43) | 5976 | 51.5 | 3.08 (2.95-3.22) | 6674 | 55.7 | 3.72 (3.56-3.89) |
| Visits by smoker | 2775 | 1729 | 1890 | 1891 | 2117 | ||||||||||
| % of patients advised to quit | 1243 | 44.8 | – | 1430 | 82.7 | 4.71 (4.06-5.48) | 1490 | 78.8 | 3.72 (3.23-4.28) | 1589 | 84.0 | 5.16 (4.44-6.00) | 1877 | 88.7 | 7.76 (6.61-9.12) |
| % of patients assessed for readiness to quit | 438 | 15.8 | – | 1283 | 74.2 | 14.90 (12.80-17.33) | 1244 | 65.8 | 9.87 (8.56-11.39) | 1225 | 64.8 | 9.29 (8.08-10.69) | 1164 | 55.0 | 6.15 (5.37-7.03) |
| Visits by smoker ready to quit in 30 d | 184 | 484 | 437 | 399 | 301 | ||||||||||
| % of patients who accepted referral | 1 | 0.5 | – | 285 | 58.9 | 260.51 (36.87-1840.52) | 245 | 56.1 | 230.97 (32.66-1633.34) | 151 | 37.8 | 110.17 (15.55-780.34) | 93 | 30.9 | 80.95 (11.38-575.80) |
Abbreviations: AAC, Ask-Advise-Connect; OR, odds ratio.
aEach time point represents 30 days of data (eg, the 1 month after starting AAC represents day 1 to day 30 postintervention).
Table 6Frequency of Tobacco Use Assessment; Provision of Advice to Quit; Offer of Referral for Cessation Counseling by AAC Time Period; and Test of HTE for Sex, Age, Race, Ethnicity, and Insurance Type
| Description | Pre-AAC implementation (n = 36 676), % | Post–AAC-only implementation (n = 139 384), % | Pre-AAC to post–AAC-only difference | Significant characteristic × time period interaction effect |
|---|---|---|---|---|
| Aska, % yes | ||||
| Sex | ||||
| Male | 24.1 | 52.6 | 28.5 | <0.001 |
| Female | 27.6 | 49.9 | 22.3 | |
| Age, y | ||||
| 18-34 | 22.6 | 43.1 | 20.5 | |
| 35-64 | 27.8 | 53.1 | 26.0 | |
| ≥65 | 27.9 | 53.7 | 25.8 | |
| Race | ||||
| White | 33.4 | 56.5 | 23.1 | <0.001b |
| Black | 21.6 | 48.7 | 27.1 | |
| Other | 22.3 | 34.2 | 11.9 | |
| Ethnicity | ||||
| Non-Hispanic | 27.7 | 52.7 | 25.0 | |
| Hispanic | 18.4 | 35.9 | 17.5 | |
| Primary insurance class | ||||
| Commercial | 29.8 | 52.2 | 22.4 | <0.001 |
| Medicaid/self-pay | 23.5 | 47.6 | 24.1 | |
| Medicare | 28.6 | 55.2 | 26.6 | |
| Advise, % yes | ||||
| Sex | ||||
| Male | 22.2 | 40.9 | 18.7 | |
| Female | 12.9 | 30.4 | 17.5 | |
| Age, y | ||||
| 18-34 | 15.3 | 30.3 | 15.0 | |
| 35-64 | 17.4 | 38.3 | 20.9 | |
| ≥65 | 9.4 | 23.1 | 13.7 | |
| Race | ||||
| White | 16.1 | 36.2 | 20.1 | |
| Black | 16.3 | 32.9 | 16.6 | |
| Other | 5.7 | 21.4 | 15.7 | |
| Ethnicity | ||||
| Non-Hispanic | 15.8 | 34.7 | 18.9 | |
| Hispanic | 11.8 | 22.7 | 10.9 | |
| Primary insurance class | ||||
| Commercial | 12.2 | 28.1 | 15.9 | |
| Medicaid/self-pay | 20.7 | 41.1 | 20.4 | |
| Medicare | 12.5 | 29.8 | 17.3 | |
| Assess readiness, % yes | ||||
| Sex | ||||
| Male | 6.4 | 26.8 | 20.4 | |
| Female | 4.2 | 18.7 | 14.5 | |
| Age, y | ||||
| 18-34 | 4.8 | 17.5 | 12.7 | |
| 35-64 | 5.5 | 25.4 | 19.9 | |
| ≥65 | 2.4 | 12.5 | 10.1 | |
| Race | ||||
| White | 4.4 | 21.1 | 16.7 | |
| Black | 5.9 | 23.5 | 17.6 | |
| Other | 1.2 | 7.7 | 6.5 | |
| Ethnicity | ||||
| Non-Hispanic | 5.1 | 22.0 | 16.9 | |
| Hispanic | 2.6 | 13.7 | 11.1 | |
| Primary insurance class | ||||
| Commercial | 2.7 | 15.3 | 12.6 | |
| Medicaid/self-pay | 7.3 | 28.3 | 21.0 | |
| Medicare | 4.2 | 18.2 | 14.0 | |
| Accept connect, % yes | ||||
| Sex | ||||
| Male | 0 | 43.7 | 43.7 | |
| Female | 0.8 | 43.6 | 42.8 | |
| Age, y | ||||
| 18-34 | 0 | 41.5 | 41.5 | |
| 35-64 | 0 | 44.6 | 44.6 | |
| ≥65 | 12.5 | 40.0 | 27.5 | |
| Race | ||||
| White | 0 | 40.4 | 40.4 | |
| Black | 0.7 | 45.8 | 45.1 | |
| Other | 0 | 48.1 | 48.1 | |
| Ethnicity | ||||
| Non-Hispanic | 0.6 | 42.9 | 42.3 | |
| Hispanic | 0 | 52.0 | 52.0 | |
| Primary insurance class | ||||
| Commercial | 0 | 41.8 | 41.8 | |
| Medicaid/self-pay | 0 | 44.1 | 44.1 | |
| Medicare | 3.0 | 42.9 | 39.9 | |
Abbreviations: AAC, Ask-Advise-Connect; HTE, heterogeneity of treatment effect.
aAnalyses for “Ask” adjusted for smoking status.
bIndicates significant interaction effect at P < .001 and >10% difference in differences. Only statistically significant values are indicated in this table.
Table 7Clinician Characteristics
| Characteristic | Received TMCP training (n = 44), No. (%) | No TMCP training (n = 16), No. (%) | P value |
|---|---|---|---|
| Sex | |||
| Male | 19 (43) | 1 (6) | .001 |
| Female | 25 (57) | 15 (94) | |
| Training | |||
| MD | 30 (68) | 7 (44) | |
| DO | 5 (11) | 1 (6) | .09 |
| NP | 9 (21) | 7 (44) | |
| PA | 0 (0) | 1 (6) | |
| Specialty | |||
| Family medicine | 25 (57) | 7 (44) | |
| Internal medicine | 19 (43) | 9 (56) | .37 |
| No. of y since residency, mean (SD) | 16.3 (11.0) | 11.4 (9.4) | .12 |
Abbreviations: DO, doctor of osteopathic medicine; MD, doctor of medicine; NP, nurse practitioner; PA, physician assistant; TMCP, Teachable Moment Communication Process.
Table 8Characteristics of Patient Visits for the Pre-AAC, AAC-Only, and AAC+TMCP Implementation Periods
| Description | Overall (N = 224 079), No. (%) | Pre-AAC (n = 34 104), No. (%) | AAC-only (n = 121 518), No. (%) | AAC+TMCP (n = 68 457), No. (%) |
|---|---|---|---|---|
| Sex | ||||
| Male | 68 967 (30.8) | 10 624 (31.2) | 37 768 (31.1) | 20 575 (30.1) |
| Female | 155 097 (69.2) | 23 479 (68.8) | 83 736 (68.9) | 47 882 (69.9) |
| Age, y | ||||
| 18-34 | 55 665 (24.8) | 8534 (25.0) | 29 834 (24.6) | 17 297 (25.3) |
| 35-64 | 127 436 (56.9) | 19 872 (58.3) | 69 551 (57.2) | 38 013 (55.5) |
| ≥65 | 40 964 (18.3) | 5698 (16.7) | 22 119 (18.2) | 13 147 (19.2) |
| Race | ||||
| White | 100 639 (50.2) | 15 700 (51.5) | 54 089 (50.5) | 30 850 (49.2) |
| Black | 90 431 (45.1) | 13 707 (44.9) | 48 851 (45.6) | 27 873 (44.4) |
| Other | 9333 (4.7) | 1099 (3.6) | 4206 (3.9) | 4028 (6.4) |
| Ethnicity | ||||
| Non-Hispanic | 188 206 (86.1) | 29 065 (87.4) | 101 128 (85.3) | 58 013 (86.9) |
| Hispanic | 30 340 (13.9) | 4177 (12.6) | 17 413 (14.7) | 8750 (13.1) |
| Primary insurance class | ||||
| Commercial | 64 098 (29.2) | 9932 (30.1) | 34 097 (28.7) | 20 069 (29.8) |
| Medicaid | 88 039 (40.2) | 13 567 (41.1) | 48 064 (40.4) | 26 408 (39.2) |
| Medicare | 53 054 (24.2) | 7587 (23.0) | 28 874 (24.3) | 16 593 (24.6) |
| Self-pay | 13 824 (6.3) | 1872 (5.7) | 7669 (6.5) | 4283 (6.4) |
| Other | 243 (0.1) | 32 (0.1) | 142 (0.1) | 69 (0.1) |
| Smoking status | ||||
| Current smoker | 47 533 (25.9) | 7600 (26.3) | 25 913 (21.3) | 14 020 (25.5) |
| Former smoker | 51 416 (28.0) | 8003 (27.7) | 27 832 (22.9) | 15 581 (28.3) |
| Never smoked | 84 536 (46.1) | 13 243 (45.9) | 45 849 (37.7) | 25 444 (46.2) |
| Not assesseda | 40 580 (18.1) | 5258 (15.4) | 21 910 (18.0) | 13 412 (19.6) |
| Readiness to quit assessed | ||||
| No | 14 966 (43.9) | 2242 (84.1) | 7082 (36.5) | 5642 (47.0) |
| Yes | 19 121 (56.1) | 425 (15.9) | 12 337 (63.5) | 6359 (53.0) |
| Ready to quit | ||||
| No | 13 752 (71.9) | 246 (57.9) | 8607 (69.8) | 4899 (77.0) |
| Yes | 5369 (28.1) | 179 (42.1) | 3730 (30.2) | 1460 (23.0) |
| MetroHealth center | ||||
| 1 | 16 410 (7.3) | 3003 (8.8) | 7665 (6.3) | 5742 (8.4) |
| 2 | 45 620 (20.4) | 5899 (17.3) | 25697 (21.1) | 14024 (20.5) |
| 3 | 22 481 (10.0) | 3727 (10.9) | 9224 (7.6) | 9530 (13.9) |
| 4 | 26 980 (12.0) | 4521 (13.3) | 14777 (12.2) | 7682 (11.2) |
| 5 | 34 217 (15.3) | 4680 (13.7) | 21073 (17.3) | 8464 (12.4) |
| 6 | 43 023 (19.2) | 6862 (20.1) | 22855 (18.8) | 13306 (19.4) |
| 7 | 28 697 (12.8) | 4399 (12.9) | 17011 (14.0) | 7287 (10.6) |
| 8 | 6637 (3.0) | 1013 (3.0) | 3202 (2.6) | 2422 (3.5) |
Abbreviations: AAC, Ask-Advise-Connect; TMCP, Teachable Moment Communication Process.
aNot included in the denominator for other smoking status.
Table 9Evaluation of the Added Effect of the TMCP Training on Tobacco-Cessation Support Outcomes
| 1-3 mo before AAC | AAC only | AAC+TMCPa | AAC+TMCP among clinicians who used the document flow sheet | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | OR (95% CI) | No. | % | OR (95% CI) | No. | % | OR (95% CI) | No. | % | OR (95% CI)b | |
| Total visits | 20 469 | 77 500 | 41 774 | 661 | ||||||||
| % of patients asked | 5618 | 27.5 | – | 38 687 | 49.9 | 2.76 (2.67-2.86) | 26 452 | 63.3 | 1.73 (1.70-1.77) | 492 | 74.4 | 1.89 (1.62-2.20) |
| Visits by smokers | 1744 | 13 342 | 8198 | 466 | ||||||||
| % of visits in which patients were advised | 778 | 44.6 | – | 11 323 | 84.9 | 4.35 (3.79-5.00) | 7466 | 91.1 | 1.97 (1.81-2.14) | 443 | 95.1 | 3.21 (2.19-4.72) |
| % of visits in which patients were assessed for readiness to quit | 226 | 13.0 | – | 8848 | 66.3 | 12.34 (10.67-14.27) | 4684 | 57.1 | 0.66 (0.62-0.69) | 347 | 74.5 | 1.24 (1.00-1.54) |
| Ready to quit (No. of visits) | 115 | 2690 | 1073 | 93 | ||||||||
| Acceptance of referral during visits | ||||||||||||
| % of visits in which patient accepted a referral | 1 | 0.9 | – | 1176 | 43.7 | 83.20 (12.58-550.36) | 369 | 34.4 | 0.66 (0.57-0.77) | 43 | 46.2 | 1.00 (0.66-1.51) |
| % of visits in which patient accepted QL referral | 0 | 0.0 | – | 827 | 30.7 | NA | 252 | 23.5 | 0.68 (0.58-0.80) | 22 | 23.7 | 0.71 (0.45-1.14) |
| % of visits in which patient accepted FFS referral | 1 | 0.9 | – | 349 | 13.0 | 14.89 (2.50-88.70) | 117 | 10.9 | 0.79 (0.64-0.98) | 21 | 22.6 | 1.45 (0.85-2.49) |
| Ready to quit (No. of patients) | 112 | 2239 | 1001 | 93 | ||||||||
| Patients who accepted referral | 1 | 0.9 | – | 1089 | 48.6 | 77.67 (13.10-460.61) | 357 | 35.7 | 0.59 (0.47-0.74) | 43 | 46.2 | 0.80 (0.50-1.29) |
| Patients who accepted QL referral | 0 | 0.0 | – | 765 | 34.2 | NA | 247 | 24.7 | 0.64 (0.50-0.82) | 22 | 23.7 | 0.58 (0.38-0.90) |
| Patients who accepted FFS referral | 1 | 0.9 | – | 328 | 14.7 | 14.47 (2.88-72.66) | 112 | 11.2 | 0.74 (0.58-0.95) | 21 | 22.6 | 1.42 (0.86-2.34) |
| QL referrals received | 649 | 200 | 15 | |||||||||
| Contact rate | NA | 211 | 32.5 | NA | 44 | 22.0 | 0.58 (0.36-0.95) | 3 | 20.0 | 0.53 (0.18-1.55) | ||
| Enrollment rate | NA | 158 | 74.9 | NA | 29 | 65.9 | 0.65 (0.35-1.23) | 1 | 33.3 | 0.15 (0.01-1.96) | ||
| FFS referrals received | 0 | – | 276 | 106 | 21 | |||||||
| Contact rate | NA | – | 75 | 27.2 | NA | 23 | 21.7 | 0.82 (0.53-1.26) | 5 | 23.8 | 1.00 (0.46-2.18) | |
| Enrollment rate | NA | – | 50 | 66.7 | NA | 18 | 78.3 | 1.74 (0.47-6.40) | 3 | 60.0 | 0.79 (0.12-5.18) | |
| Medications | 113 | 7.9 | – | 1126 | 14.9 | 1.85 (1.53-2.23) | 718 | 12.4 | 0.81 (0.74-0.88) | 139 | 31.6 | 2.61 (1.92-3.55) |
| Quit attemptsc | 51 | 2.5 | – | 44 | 2.1 | 0.82 (0.54-1.23) | 45 | 2.3 | 1.09 (0.72-1.67) | 8 | 3.7 | 1.95 (0.86-4.45) |
Abbreviations: AAC, Ask-Advise-Connect; FFS, Freedom From Smoking; NA, not applicable; QL, quitline; TMCP, Teachable Moment Communication Process.
aComparison is the post-TMCP to post-AAC period among clinicians who received training.
bComparison is post-TMCP for visits in which a document flow sheet was used compared with visits during the post-AAC period for clinicians who had ever used the document flow sheet.
cQuit attempts are defined based on ≥2 visits in the time period, a change in smoking status from current smoker to former smoker, and documentation of a quit date. Numerator and denominator for quit attempts for each period: 51/1986 (1-3 mo before AAC), 44/2088 (AAC only), 45/1951 (AAC+TMCP), and 8/216 (AAC+TMCP among clinicians who used the document flow sheet).
Table 10Patient-Reported Outcomes of Being Treated With Respect, Feeling Listened to, and Stating That the Discussion About Smoking Was Helpful
| 1-3 mo before AAC (N = 171 surveys) | AAC only (N = 169 surveys) | AAC+TMCP (N = 164 surveys) | |
|---|---|---|---|
| Did the nurse talk about smoking? | |||
| % of patients responding “yes” | 65 | 72 | 70 |
| Mean (SD)a | Mean (SD)a | Mean (SD)a | |
| I was treated with respect | 4.35 (0.91) | 4.53 (0.76) | 4.37 (0.92) |
| I felt listened to | 4.17 (1.00) | 4.36 (0.84) | 4.29 (0.90) |
| I was able to honestly speak my mind about quitting | 4.09 (1.14) | 4.28 (0.98) | 4.23 (1.04) |
| My opinion about quitting smoking was treated with respect | 4.08 (1.06) | 4.36 (0.98) | 4.28 (1.00) |
| Things were explained in a way that I could understand | 4.12 (1.07) | 4.36 (0.96) | 4.27 (0.95) |
| Did the doctor talk about smoking? | |||
| % of patients responding “yes” | 79 | 74 | 75 |
| Mean (SD)a | Mean (SD)a | Mean (SD)a | |
| I was treated with respect | 4.43 (0.86) | 4.55 (0.85) | 4.58 (0.75) |
| I felt listened to | 4.37 (0.93) | 4.45 (0.90) | 4.44 (0.87) |
| I was able to honestly speak my mind about quitting | 4.37 (0.92) | 4.38 (0.99) | 4.48 (0.82) |
| My opinion about quitting smoking was treated with respect | 4.29 (0.92) | 4.36 (0.97) | 4.36 (0.86) |
| Things were explained in a way that I could understand | 4.32 (0.86) | 4.37 (1.03) | 4.51 (0.73) |
| The discussion about smoking was helpful | 4.15 (0.99) | 4.13 (1.26) | 4.33 (0.92) |
Abbreviations: AAC, Ask-Advise-Connect; TMCP, Teachable Moment Communication Process.
aItems are reported on a 5-point Likert scale (1 = poor, 2 = fair, 3 = good, 4 = very good, 5 = excellent).
Table 11Interviewee Sample Characteristics
| Characteristic | Total (N = 229) | Participants (n = 55) | Nonparticipants (n = 115) | Not contacteda (n = 59) |
|---|---|---|---|---|
| Age, y, mean (SD) | 51.3 (13.2) | 50.6 (12.0) | 51.6 (13.8) | 51.6 (13.4) |
| Female, % | 65 | 76 | 57 | 71 |
| Black, % | 54 | 58 | 55 | 49 |
| Insurance type, % | ||||
| Commercial | 3 | 2 | 3 | 4 |
| Medicaid | 64 | 57 | 65 | 67 |
| Medicare | 33 | 39 | 32 | 28 |
| Self-pay | 1 | 2 | 0 | 2 |
aThese individuals were sampled but were not invited to participate because the target number of interviews was completed before we attempted to contact this group.
Table 12Themes and Example Quotations From Smokers e-Referred to the QL
| Differing expectations regarding the QL referral |
|---|
| “You know, and I just wasn't sure [what to expect]. To be honest with you, I thought it was a program like a friend of mine went through some years ago where he actually went to one of the clinics in the evenings.” [ID #08] |
| “The only thing I got was ‘You call them, and they'll call you back.’ That's the only thing I got…. I wanted to ask, but I was in pain. I couldn't really breathe and stuff like that. I wanted to know about, like, if you go to the hospital and you sit down with somebody. I wanted to ask those questions, but I honestly was not feeling good and I didn't wanna hear anything.” [ID #14] |
| “I wanted patches. So that's what I thought I was gonna get, some patches. Like I was saying, that's not what I wanted to do—them checking on me and this and that and all that…. So you know, I told them, ‘That's all right.’ If I couldn't get the patches, that's all right. That was the end of that conversation.” [ID #25]. |
| Changing life circumstances and stressors |
| “My mother had a stroke, and she wasn't doing too good and wasn't nobody here to take care of her but me. You know how hard that was on me. She was bedridden, and she died like in August of last year. Yeah, and trying to take care of myself and trying to take care of her. It was a lot.” |
| “I just had a lot going on. A lot of issues with family, things going on lately. Our home was just burglarized a couple weeks ago. I've just had a lot going on…. I mean it's definitely something I'd like to do eventually. It's just gotta be the right time, and a lot of times when you try to quit something and then you go back to it, becomes, you know it just becomes worse, I guess.” [ID #33] |
| “Well they sent me the brochures and everything. But my mindset wasn't in the right frame of mind at that point, ‘cause my dad was in a nursing home. And he just recently passed away, so I wasn't really in the right state of mind back then. It was kind of stressful, and that was like the stress-relief to get out the nursing home and have me a cigarette and go home.” [ID #44] |
| Unable to find time for counseling |
| “I've been at work so much that I never get a chance to conversate with them, ‘cause I'm at work like from morning ‘til late evening.” [ID #13] |
| “I actually received a couple calls that I missed because I was at appointments, or I was either at my kids' school or something and didn't answer the phone.” [ID #12] |
| “Usually when they call, sometimes I don't answer because I'm either picking up my kid, or taking him to school … and then with Christmas, holidays. Everything is just, you know, and then trying to figure out with the doctor, ‘cause well I just had another episode, so I was in the hospital.” [ID #22] |
| Cell phone barriers |
| “They probably tried to call me, but my phone's been stupid…. I cracked it, so sometimes it answers and sometimes it doesn't.” [ID #54] |
| “Yeah, and then the phone I had, I lost it, and I ain't been able to afford me another phone, but I got a birthday soon coming. I guess they'll pitch in and buy me a phone, and I'm using a temporary phone now until I get me another real good phone. I had phones and kept having problems with them.” [ID #52] |
| “The MA asked if I had any interest in stopping and I told her yes, and then she gave me or told me about the quitline was supposed to call me, which I believe they may have, but the number comes up and if it's an 800 number, I usually don't answer it, because you know it doesn't come up under the quitline, you know, ID. It just comes up as an 800 number.” [ID #04] |
| Discomfort with/disbelief in the efficacy of QL counseling |
| “I did [agree to be connected to the quitline], and we did speak. Someone did call me with the department of the quitline, and I was not comfortable. I'm not gonna lie to you…. I think it was just the person that spoke to me over the phone. In reality, I know that it's just your job to try to give information out, or try to help someone, but you need to feel comfortable with somebody when you speak to them over the phone, and I just didn't feel comfortable with the first call I got. So I didn't agree to the over-the-phone line quitting situation because, I don't know. I just didn't feel comfortable.” [ID #15] |
| “You know the first time, the lady was professional and generous. It's just, I don't think it was very helpful to me.”[ID #41] |
| “Cause talking with somebody about quitting doesn't do any good. I feel like talking wouldn't do any good. ‘Cause I would go ahead on and say ‘Yeah. Um hmm. Yeah. You're right. You're right,' and it'll be going in one ear, coming out the other. [ID #23] |
| Quitting on their own |
| “I got a call and they asked me, you know they said ‘Are you interested in quitting?’ and I said ‘Yeah.’ I told them I was in the process of trying to quit then, you know, and they told me if I needed help, to get in touch with them.” [ID #28] |
| “They called me, but I didn't really speak with them because I actually stopped, and I didn't need the help. And I've been doing good ever since then. “[ID #03] |
Abbreviation: QL, quitline.
Suggested citation:
Flocke S, Seeholzer E, Love T, et al. (2021). Adding a Teachable Moment Approach to a Team-Based Primary Care Ask-Advise-Connect Approach for Tobacco Cessation. Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/02.2021.IHS.150329879IC
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.