This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-.

StatPearls [Internet].
Show detailsContinuing Education Activity
Extranodal NK/T-cell lymphoma, nasal type, is a rare subtype of non-Hodgkin lymphoma. Patients commonly present with a nasal mass, nasal obstruction, or nasal bleeding. With the application of modern therapies, clinical outcomes of patients with this disease have improved significantly. This activity describes the evaluation and management of extranodal NK/T-cell lymphoma, nasal type, and reviews the role of the interprofessional team in evaluating and treating patients with this disease.
Objectives:
- Identify the etiology of extranodal NK/T-cell lymphoma.
- Describe the evaluation of extranodal NK/T-cell lymphoma.
- Outline the management options available for extranodal NK/T-cell lymphoma.
Introduction
Extranodal NK/T-cell lymphoma, nasal type, is a rare subtype of non-Hodgkin lymphoma, characterized by a universal association with Epstein-Barr virus (EBV), and disfiguring clinical presentations.[1][2][3] With the application of modern therapies, the clinical outcomes of these patients have improved significantly.[4] Nevertheless, survival is still heavily dependent on the type of disease and stage at diagnosis.
Etiology
Irrespective of the ethnic origin of patients, there is a strong association of EBV infection in almost all cases of extranodal NK/T-cell lymphoma, suggesting an important role of the virus in the etiology of this disease.[2][5][6][7][8][9]
Epidemiology
Extranodal NK/T-cell lymphoma is regarded as a rare subtype of non-Hodgkin lymphoma.[1] The disease is more prevalent in Asian countries than in Europe and the United States.[4] It often affects adults and is more common in men than in women.
Pathophysiology
Genetic abnormalities, EBV infection, and tumor microenvironment have been shown to play significant roles in the molecular pathogenesis of extranodal NK/T-cell lymphoma.[10][11]
1. Genetic Abnormalities
JAK/STAT Pathway
The Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway is important for hematopoiesis and immune development. In extranodal NK/T-cell lymphoma, STAT3 of the JAK/STAT cascade is the most frequently mutated genes followed by TP53, JAK3, JAK1, and SOCS1.[12][13][14] STAT3 mutations promote STAT3 phosphorylation and transcriptional activity of STAT3 in the absence of cytokines. By binding to the programmed cell death-ligand 1 (PD-L1) gene promoter, activated STAT3 then upregulates the expression of PD-L1. Increased PD-L1 interacts with the programmed cell death protein 1 (PD-1), an inhibitory co-receptor on T-cells, and suppresses T-cell activation and receptor signaling.[15] This leads to T-cell exhaustion which favors tumor cell survival.
Epigenetic Dysregulation
Enhancer of zeste homolog 2 (EZH2) is a histone methyltransferase and a catalytic component of the polycomb repressive complex 2 (PRC2) molecule.[16] The PRC2 molecule is one of the polycomb group proteins that form chromatin-modifying complexes essential for embryonic development and stem cell renewal but are commonly deregulated in cancer.
EZH2 is aberrantly overexpressed in extranodal NK/T-cell lymphoma, which is mediated by MYC-induced repression of regulatory microRNAs.[17][18][19] It exerts its oncogenic function by inducing gene repression through its effect on chromatin via its histone methyltransferase activity, and by promoting the transcription of cyclin D1.
Besides, phosphorylation by JAK3 promotes EZH2 as a transcriptional activator.[20] Through the upregulation of genes involved in DNA replication, cell cycle, biosynthesis, stemness, and invasiveness, phosphorylated EZH2 promotes a higher proliferative capacity of the tumor cells.
PD-L1 Genetic Abnormalities
Structural variations and focal copy number alterations affecting PD-L1 are associated with an overexpression of PD-L1 in extranodal NK/T-cell lymphoma.[21]
Hereditary Susceptibility
A genome-wide association study found out that a single-nucleotide polymorphism located in HLA-DPB1, namely rs9277378, was associated with a 2.3 times higher risk of extranodal NK/T-cell lymphoma compared to baseline, rendering its hereditary susceptible to the disease.[22]
Other Gene Mutations
Extranodal NK/T-cell lymphoma is associated with the deletion in the chromosome 6q21-25, and mutations in the cell surface receptor FAS (Apo-1/CD95), p53, K-ras, and c-kit.[23][24][25][26][27][28][29][30][31][32][33]
2. EBV Infection
EBV infection is believed to play an important role in the pathogenesis of extranodal NK/T-cell lymphoma though its actual mechanism is still undetermined. It is known to integrate its genomic fragment into different repeat families of the human genome, such as SINE, LINE, and satellite.[34] By integrating its genomic fragment into the intron of the human non-homologous end-joining factor 1 (NHEJ1) gene, EBV downregulates NHEJ1, which is an important DNA repair factor for the NHEJ pathway that mediates repair of double-stranded breaks. This results in genome-wide instability with the onset of extranodal NK/T-cell lymphoma.
By expressing EBV nuclear antigen 1 (EBNA1), latent membrane protein (LMP) 1, LMP2A, and LMP2B (latency phase II), EBV modulates cell signaling, and forms barriers to apoptotic signals to escape from T-cell-mediated immune response.[35] Besides, the EBV BART RNAs, which are highly transcribed in extranodal NK/T-cell lymphoma, is thought to play an important role in driving the disease and immune evasion.[34]
3. Tumor Microenvironment
Chemokines play a crucial role in the proliferation of tumor cells with the recruitment of inflammatory cells.[36][37] Tumor cells secrete IP-10 (interferon gamma-induced protein 10), CCL2 (C-C motif chemokine ligand 2), and CCL22 (C-C motif chemokine ligand 22) which are monocyte-attractant chemokines. Monocytes, in turn, promote proliferation, LMP-1 expression, and IP-10 production of tumor cells via membrane-bound IL-15/IL-15 receptor alpha complex. This positive feedback loop between tumor cells and monocytes may contribute to lymphoma progression.
Histopathology
Angioinvasiveness and necrosis are distinguishing pathologic features in most cases of extranodal NK/T-cell lymphoma.[38] Surrounded by various inflammatory cells such as granulocytes, lymphocytes, monocytes, and macrophages, the lymphoma cells are usually medium-sized or a mixture of small and large cells. They usually reveal surface CD3−, cytoplasmic CD3ε+, CD56+, CD16+/−, and germline T-cell receptor (TCR) on immunophenotyping, demonstrating NK-cell lineage. In a subset of lymphoma cells of T-cell origin, positive surface CD3 and negative CD56 may be detected. EBV-encoded RNA in situ hybridization (EBER-ISH) and cytotoxic granule protein TIA-1 are always positive.
History and Physical
Most patients with extranodal NK/T-cell lymphoma are of nasal type, which is localized to the upper aerodigestive tract.[3] They commonly present with a nasal mass, nasal obstruction, or nasal bleeding. Other clinical presentations include hoarseness of voice, dysphagia, halitosis, airway obstruction, and dysphonia. Bone marrow involvement is not common in initial presentation.[39]
In patients with an extranasal involvement, skin, testis, and gastrointestinal (GI) tract are typically affected.[2][40] Abdominal pain, GI bleeding, and bowel perforation are the most common presentations in patients with GI involvement.
Evaluation
A definitive diagnosis requires an adequate biopsy with histopathological and immunohistochemical examinations. It is followed by proper staging with positron emission tomography/computed tomography (PET/CT) scans to assess the extent of the disease and to guide therapy.[41] Contrast-enhanced CT scans and magnetic resonance imaging scans can also be used. The PET/CT results are interpreted using the 2014 Lugano classification as follows:[42]
- Stage I: a single lymph node, a group of adjacent lymph nodes or single extranodal lesions without nodal involvement
- Stage II: two or more lymph node groups on the same side of the diaphragm or limited contiguous extranodal involvement
- Stage II bulky: stage II as above with a bulky feature defined as a single nodal mass of 10 centimeters or greater than a third of the transthoracic diameter
- Stage III: nodes on both sides of diaphragm or nodes above diaphragm with spleen involvement
- Stage IV: additional noncontiguous extralymphatic involvement
A useful prognostic model for patients with extranodal NK/T-cell lymphoma is the prognostic index of natural killer lymphoma (PINK), which includes age above 60 years, stage III to IV disease, distant lymph node involvement, and non-nasal disease.[43] A modified PINK model that includes EBV-DNA viral load at the time of diagnosis (PINK-E) can also be used. EBV-DNA viral load by quantitative PCR correlates well with clinical stage, treatment response, and prognosis; therefore, it plays an important role in the diagnosis and monitoring of the disease.[44][45]
Treatment / Management
The therapeutic approach of extranodal NK/T-cell lymphoma is based on several factors, such as the age of patients, the extent of disease, potential toxicities, and survivorship.
1. Induction Therapy
Nasal Disease (Stage I–II)
Patients with nasal disease (stage I–II) may be treated with concurrent chemoradiotherapy (CRT) regimens such as DeVIC (dexamethasone, etoposide, ifosfamide, and carboplatin) with radiation therapy (RT), or VIPD (etoposide, ifosfamide, cisplatin, dexamethasone) with RT.[46][47][48][49] Other regimens that may be considered include sequential CRT with modified SMILE [dexamethasone (steroid), methotrexate, ifosfamide, pegaspargase, and etoposide] with RT, and sandwich CRT with P-GEMOX (pegaspargase, gemcitabine, and oxaliplatin) with RT.[50][51][52] For patients who are unfit for chemotherapy, RT alone may be considered.[53]
Nasal Disease (Stage IV) and Extranasal Disease (Stage I–IV)
Pegaspargase-based combination chemotherapy regimens such as AspaMetDex (L-asparaginase, methotrexate, and dexamethasone), modified SMILE, or P-GEMOX, with or without RT may be considered in patients with nasal disease (stage IV) or extranasal disease (stage I–IV).[50][52][54] Other regimens that may be considered include concurrent CRT regimens such as DeVIC with RT, or VIPD with RT.[46][47][48][49]
2. Response Assessment
At the end of induction therapy, patients should undergo an evaluation with PET/CT scans, ear, nose, and throat examination, and EBV viral load to establish remission status. Results from PET/CT scans are interpreted using the Deauville criteria.[41][44][45][55]
Patients with nasal disease (stage I–II) with complete remission (disappearance of all disease) may be observed without further treatment.[56] In contrast, those with partial remission (regression of disease) should undergo a biopsy.[42] Patients with a positive biopsy should be treated as refractory disease while those with a negative biopsy may be observed without further treatment.
In patients with nasal disease (stage IV) or extranasal disease (stage I–IV), hematopoietic stem cell transplant may be considered in those with complete remission or partial remission with a negative biopsy.[57][58] However, patients with a positive biopsy should be treated as refractory disease.[42]
3. Relapsed or Refractory Disease
A diagnosis of relapsed or refractory disease must be confirmed histologically by biopsy.[42] These patients may be treated with a second-line therapy using pegaspargase-based combination chemotherapy regimens such as AspaMetDex, modified SMILE, or P-GEMOX.[50][52][54] Pembrolizumab, an anti-PD-1 monoclonal antibody, has shown remission in relapsed or refractory disease and may be considered in these patients.[59] Other treatment options include supportive care and participation in a clinical trial.[56]
Differential Diagnosis
Conditions that mimic extranodal NK/T-cell lymphoma include aggressive NK-cell leukemia, nasopharyngeal carcinoma, and other mature T- and NK-cell neoplasms. Therefore, it is crucial to have an adequate biopsy with histopathological and immunohistochemical examinations to make a definitive diagnosis.
Prognosis
Clinical outcomes of extranodal NK/T-cell lymphoma have improved significantly with the application of modern therapies.[4] Nevertheless, survival is still heavily dependent on the type of disease and stage at diagnosis. In patients with nasal disease, the 5-year overall survival rate was 54%, but it was 34% in those with extranasal disease.
Complications
The most common cause of death among patients with extranodal NK/T-cell lymphoma is non-Hodgkin lymphoma, followed by other malignant cancers, heart disease, and infection.[60] On rare occasions, patients may present with a hemophagocytic syndrome, a rare and life-threatening disease characterized by high fever, pancytopenia, hepatosplenomegaly, liver dysfunction, coagulopathy, hyperferritinemia, and hemophagocytosis in bone marrow or other organs.[2][61]
Deterrence and Patient Education
Being diagnosed with extranodal NK/T-cell lymphoma and undergoing treatment can have significant psychosocial impacts on patients and their families, as these can bring dramatic changes in their physical, spiritual, emotional, and interpersonal dimensions. Every clinic visit should include screening and assessment of emotional and social concerns of patients and their families. They should also be well-informed of the types of treatment, such as CRT and combination chemotherapy regimens, pros and cons of each treatment, side effects, supportive measures, and long-term management plan. Regular follow-up with primary care physicians and oncologists is also important to monitor treatment response and treatment-related toxicities.
Enhancing Healthcare Team Outcomes
Patient-centered teamwork approach is essential when managing patients with extranodal NK/T-cell lymphoma. An interprofessional team should work together in every step of management—treatment of the disease, prevention and management of complications related to disease or treatment, and providing comprehensive care for patients and their families, with regular follow-ups, psychosocial support, survivorship care, and in end-of-life care.
Review Questions
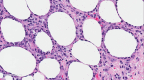
Figure
Subcutaneous Panniculitis. High-power image of subcutaneous panniculitis, like a T-cell lymphoma showing rimming of fat cells by lymphocytes with frequent apoptotic debris (400x). Contributed by D Lynch, MD
References
- 1.
- Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016 Nov 12;66(6):443-459. [PubMed: 27618563]
- 2.
- Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, Vose J, Armitage JO, Liang R., International Peripheral T-Cell Lymphoma Project. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009 Apr 23;113(17):3931-7. [PubMed: 19029440]
- 3.
- Liang R. Advances in the management and monitoring of extranodal NK/T-cell lymphoma, nasal type. Br J Haematol. 2009 Oct;147(1):13-21. [PubMed: 19604234]
- 4.
- Fox CP, Civallero M, Ko YH, Manni M, Skrypets T, Pileri S, Kim SJ, Cabrera ME, Shustov AR, Chiattone CS, Horwitz SM, Dlouhy I, Spina M, Hitz F, Montoto S, Nagler A, Martinez V, De Souza CA, Fernandez-Alvarez R, Ballova V, Gabús R, Inghirami G, Federico M, Kim WS. Survival outcomes of patients with extranodal natural-killer T-cell lymphoma: a prospective cohort study from the international T-cell Project. Lancet Haematol. 2020 Apr;7(4):e284-e294. [PubMed: 32105608]
- 5.
- Harabuchi Y, Yamanaka N, Kataura A, Imai S, Kinoshita T, Mizuno F, Osato T. Epstein-Barr virus in nasal T-cell lymphomas in patients with lethal midline granuloma. Lancet. 1990 Jan 20;335(8682):128-30. [PubMed: 1967431]
- 6.
- Harabuchi Y, Imai S, Wakashima J, Hirao M, Kataura A, Osato T, Kon S. Nasal T-cell lymphoma causally associated with Epstein-Barr virus: clinicopathologic, phenotypic, and genotypic studies. Cancer. 1996 May 15;77(10):2137-49. [PubMed: 8640683]
- 7.
- Kanavaros P, Lescs MC, Brière J, Divine M, Galateau F, Joab I, Bosq J, Farcet JP, Reyes F, Gaulard P. Nasal T-cell lymphoma: a clinicopathologic entity associated with peculiar phenotype and with Epstein-Barr virus. Blood. 1993 May 15;81(10):2688-95. [PubMed: 8387835]
- 8.
- Medeiros LJ, Jaffe ES, Chen YY, Weiss LM. Localization of Epstein-Barr viral genomes in angiocentric immunoproliferative lesions. Am J Surg Pathol. 1992 May;16(5):439-47. [PubMed: 1318000]
- 9.
- Weiss LM, Gaffey MJ, Chen YY, Frierson HF. Frequency of Epstein-Barr viral DNA in "Western" sinonasal and Waldeyer's ring non-Hodgkin's lymphomas. Am J Surg Pathol. 1992 Feb;16(2):156-62. [PubMed: 1310241]
- 10.
- Somasundaram N, Lim JQ, Ong CK, Lim ST. Pathogenesis and biomarkers of natural killer T cell lymphoma (NKTL). J Hematol Oncol. 2019 Mar 15;12(1):28. [PMC free article: PMC6420729] [PubMed: 30876435]
- 11.
- Harabuchi Y, Takahara M, Kishibe K, Nagato T, Kumai T. Extranodal Natural Killer/T-Cell Lymphoma, Nasal Type: Basic Science and Clinical Progress. Front Pediatr. 2019;7:141. [PMC free article: PMC6476925] [PubMed: 31041299]
- 12.
- Song TL, Nairismägi ML, Laurensia Y, Lim JQ, Tan J, Li ZM, Pang WL, Kizhakeyil A, Wijaya GC, Huang DC, Nagarajan S, Chia BK, Cheah D, Liu YH, Zhang F, Rao HL, Tang T, Wong EK, Bei JX, Iqbal J, Grigoropoulos NF, Ng SB, Chng WJ, Teh BT, Tan SY, Verma NK, Fan H, Lim ST, Ong CK. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood. 2018 Sep 13;132(11):1146-1158. [PMC free article: PMC6148343] [PubMed: 30054295]
- 13.
- Coppo P, Gouilleux-Gruart V, Huang Y, Bouhlal H, Bouamar H, Bouchet S, Perrot C, Vieillard V, Dartigues P, Gaulard P, Agbalika F, Douay L, Lassoued K, Gorin NC. STAT3 transcription factor is constitutively activated and is oncogenic in nasal-type NK/T-cell lymphoma. Leukemia. 2009 Sep;23(9):1667-78. [PMC free article: PMC2796333] [PubMed: 19421230]
- 14.
- Sim SH, Kim S, Kim TM, Jeon YK, Nam SJ, Ahn YO, Keam B, Park HH, Kim DW, Kim CW, Heo DS. Novel JAK3-Activating Mutations in Extranodal NK/T-Cell Lymphoma, Nasal Type. Am J Pathol. 2017 May;187(5):980-986. [PubMed: 28284718]
- 15.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704. [PMC free article: PMC10637733] [PubMed: 18173375]
- 16.
- Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006 May 01;20(9):1123-36. [PMC free article: PMC1472472] [PubMed: 16618801]
- 17.
- Sander S, Bullinger L, Klapproth K, Fiedler K, Kestler HA, Barth TF, Möller P, Stilgenbauer S, Pollack JR, Wirth T. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008 Nov 15;112(10):4202-12. [PubMed: 18713946]
- 18.
- Ng SB, Selvarajan V, Huang G, Zhou J, Feldman AL, Law M, Kwong YL, Shimizu N, Kagami Y, Aozasa K, Salto-Tellez M, Chng WJ. Activated oncogenic pathways and therapeutic targets in extranodal nasal-type NK/T cell lymphoma revealed by gene expression profiling. J Pathol. 2011 Mar;223(4):496-510. [PubMed: 21294123]
- 19.
- Yan J, Ng SB, Tay JL, Lin B, Koh TL, Tan J, Selvarajan V, Liu SC, Bi C, Wang S, Choo SN, Shimizu N, Huang G, Yu Q, Chng WJ. EZH2 overexpression in natural killer/T-cell lymphoma confers growth advantage independently of histone methyltransferase activity. Blood. 2013 May 30;121(22):4512-20. [PubMed: 23529930]
- 20.
- Yan J, Li B, Lin B, Lee PT, Chung TH, Tan J, Bi C, Lee XT, Selvarajan V, Ng SB, Yang H, Yu Q, Chng WJ. EZH2 phosphorylation by JAK3 mediates a switch to noncanonical function in natural killer/T-cell lymphoma. Blood. 2016 Aug 18;128(7):948-58. [PubMed: 27297789]
- 21.
- Kataoka K, Miyoshi H, Sakata S, Dobashi A, Couronné L, Kogure Y, Sato Y, Nishida K, Gion Y, Shiraishi Y, Tanaka H, Chiba K, Watatani Y, Kakiuchi N, Shiozawa Y, Yoshizato T, Yoshida K, Makishima H, Sanada M, Onozawa M, Teshima T, Yoshiki Y, Ishida T, Suzuki K, Shimada K, Tomita A, Kato M, Ota Y, Izutsu K, Demachi-Okamura A, Akatsuka Y, Miyano S, Yoshino T, Gaulard P, Hermine O, Takeuchi K, Ohshima K, Ogawa S. Frequent structural variations involving programmed death ligands in Epstein-Barr virus-associated lymphomas. Leukemia. 2019 Jul;33(7):1687-1699. [PMC free article: PMC6755969] [PubMed: 30683910]
- 22.
- Li Z, Xia Y, Feng LN, Chen JR, Li HM, Cui J, Cai QQ, Sim KS, Nairismägi ML, Laurensia Y, Meah WY, Liu WS, Guo YM, Chen LZ, Feng QS, Pang CP, Chen LJ, Chew SH, Ebstein RP, Foo JN, Liu J, Ha J, Khoo LP, Chin ST, Zeng YX, Aung T, Chowbay B, Diong CP, Zhang F, Liu YH, Tang T, Tao M, Quek R, Mohamad F, Tan SY, Teh BT, Ng SB, Chng WJ, Ong CK, Okada Y, Raychaudhuri S, Lim ST, Tan W, Peng RJ, Khor CC, Bei JX. Genetic risk of extranodal natural killer T-cell lymphoma: a genome-wide association study. Lancet Oncol. 2016 Sep;17(9):1240-7. [PMC free article: PMC6790270] [PubMed: 27470079]
- 23.
- Tien HF, Su IJ, Tang JL, Liu MC, Lee FY, Chen YC, Chuang SM. Clonal chromosomal abnormalities as direct evidence for clonality in nasal T/natural killer cell lymphomas. Br J Haematol. 1997 Jun;97(3):621-5. [PubMed: 9207410]
- 24.
- Wong KF, Chan JK, Kwong YL. Identification of del(6)(q21q25) as a recurring chromosomal abnormality in putative NK cell lymphoma/leukaemia. Br J Haematol. 1997 Sep;98(4):922-6. [PubMed: 9326190]
- 25.
- Siu LL, Chan V, Chan JK, Wong KF, Liang R, Kwong YL. Consistent patterns of allelic loss in natural killer cell lymphoma. Am J Pathol. 2000 Dec;157(6):1803-9. [PMC free article: PMC1885756] [PubMed: 11106552]
- 26.
- Sun HS, Su IJ, Lin YC, Chen JS, Fang SY. A 2.6 Mb interval on chromosome 6q25.2-q25.3 is commonly deleted in human nasal natural killer/T-cell lymphoma. Br J Haematol. 2003 Aug;122(4):590-9. [PubMed: 12899714]
- 27.
- Takakuwa T, Dong Z, Nakatsuka S, Kojya S, Harabuchi Y, Yang WI, Nagata S, Aozasa K. Frequent mutations of Fas gene in nasal NK/T cell lymphoma. Oncogene. 2002 Jul 11;21(30):4702-5. [PubMed: 12096347]
- 28.
- Shen L, Liang AC, Lu L, Au WY, Kwong YL, Liang RH, Srivastava G. Frequent deletion of Fas gene sequences encoding death and transmembrane domains in nasal natural killer/T-cell lymphoma. Am J Pathol. 2002 Dec;161(6):2123-31. [PMC free article: PMC1850920] [PubMed: 12466128]
- 29.
- Takahara M, Kishibe K, Bandoh N, Nonaka S, Harabuchi Y. P53, N- and K-Ras, and beta-catenin gene mutations and prognostic factors in nasal NK/T-cell lymphoma from Hokkaido, Japan. Hum Pathol. 2004 Jan;35(1):86-95. [PubMed: 14745729]
- 30.
- Hongyo T, Hoshida Y, Nakatsuka S, Syaifudin M, Kojya S, Yang WI, Min YH, Chan H, Kim CH, Harabuchi Y, Himi T, Inuyama M, Aozasa K, Nomura T. p53, K-ras, c-kit and beta-catenin gene mutations in sinonasal NK/T-cell lymphoma in Korea and Japan. Oncol Rep. 2005 Feb;13(2):265-71. [PubMed: 15643509]
- 31.
- Li T, Hongyo T, Syaifudin M, Nomura T, Dong Z, Shingu N, Kojya S, Nakatsuka S, Aozasa K. Mutations of the p53 gene in nasal NK/T-cell lymphoma. Lab Invest. 2000 Apr;80(4):493-9. [PubMed: 10780666]
- 32.
- Hoshida Y, Hongyo T, Jia X, He Y, Hasui K, Dong Z, Luo WJ, Ham MF, Nomura T, Aozasa K. Analysis of p53, K-ras, c-kit, and beta-catenin gene mutations in sinonasal NK/T cell lymphoma in northeast district of China. Cancer Sci. 2003 Mar;94(3):297-301. [PMC free article: PMC11160272] [PubMed: 12824925]
- 33.
- Quintanilla-Martinez L, Kremer M, Keller G, Nathrath M, Gamboa-Dominguez A, Meneses A, Luna-Contreras L, Cabras A, Hoefler H, Mohar A, Fend F. p53 Mutations in nasal natural killer/T-cell lymphoma from Mexico: association with large cell morphology and advanced disease. Am J Pathol. 2001 Dec;159(6):2095-105. [PMC free article: PMC1850589] [PubMed: 11733360]
- 34.
- Peng RJ, Han BW, Cai QQ, Zuo XY, Xia T, Chen JR, Feng LN, Lim JQ, Chen SW, Zeng MS, Guo YM, Li B, Xia XJ, Xia Y, Laurensia Y, Chia BKH, Huang HQ, Young KH, Lim ST, Ong CK, Zeng YX, Bei JX. Genomic and transcriptomic landscapes of Epstein-Barr virus in extranodal natural killer T-cell lymphoma. Leukemia. 2019 Jun;33(6):1451-1462. [PMC free article: PMC6756073] [PubMed: 30546078]
- 35.
- Gru AA, Haverkos BH, Freud AG, Hastings J, Nowacki NB, Barrionuevo C, Vigil CE, Rochford R, Natkunam Y, Baiocchi RA, Porcu P. The Epstein-Barr Virus (EBV) in T Cell and NK Cell Lymphomas: Time for a Reassessment. Curr Hematol Malig Rep. 2015 Dec;10(4):456-67. [PMC free article: PMC4679542] [PubMed: 26449716]
- 36.
- Moriai S, Takahara M, Ogino T, Nagato T, Kishibe K, Ishii H, Katayama A, Shimizu N, Harabuchi Y. Production of interferon-{gamma}-inducible protein-10 and its role as an autocrine invasion factor in nasal natural killer/T-cell lymphoma cells. Clin Cancer Res. 2009 Nov 15;15(22):6771-9. [PubMed: 19887486]
- 37.
- Kumai T, Nagato T, Kobayashi H, Komabayashi Y, Ueda S, Kishibe K, Ohkuri T, Takahara M, Celis E, Harabuchi Y. CCL17 and CCL22/CCR4 signaling is a strong candidate for novel targeted therapy against nasal natural killer/T-cell lymphoma. Cancer Immunol Immunother. 2015 Jun;64(6):697-705. [PMC free article: PMC4648286] [PubMed: 25754123]
- 38.
- Pongpruttipan T, Sukpanichnant S, Assanasen T, Wannakrairot P, Boonsakan P, Kanoksil W, Kayasut K, Mitarnun W, Khuhapinant A, Bunworasate U, Puavilai T, Bedavanija A, Garcia-Herrera A, Campo E, Cook JR, Choi J, Swerdlow SH. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 2012 Apr;36(4):481-99. [PubMed: 22314189]
- 39.
- Wong KF, Chan JK, Cheung MM, So JC. Bone marrow involvement by nasal NK cell lymphoma at diagnosis is uncommon. Am J Clin Pathol. 2001 Feb;115(2):266-70. [PubMed: 11211616]
- 40.
- Kim SJ, Jung HA, Chuang SS, Hong H, Guo CC, Cao J, Hong XN, Suzuki R, Kang HJ, Won JH, Chng WJ, Kwong YL, Suh C, Song YQ, Zhu J, Tay K, Lim ST, Suzumiya J, Lin TY, Kim WS., Asia Lymphoma Study Group. Extranodal natural killer/T-cell lymphoma involving the gastrointestinal tract: analysis of clinical features and outcomes from the Asia Lymphoma Study Group. J Hematol Oncol. 2013 Nov 16;6:86. [PMC free article: PMC4225665] [PubMed: 24238138]
- 41.
- Ding JJ, Chen YL, Zhou SH, Zhao K. Positron emission tomography/computed tomography in the diagnosis, staging, and prognostic evaluation of natural killer/T-cell lymphoma. J Int Med Res. 2018 Dec;46(12):4920-4929. [PMC free article: PMC6300951] [PubMed: 30328364]
- 42.
- Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA., Alliance, Australasian Leukaemia and Lymphoma Group. Eastern Cooperative Oncology Group. European Mantle Cell Lymphoma Consortium. Italian Lymphoma Foundation. European Organisation for Research. Treatment of Cancer/Dutch Hemato-Oncology Group. Grupo Español de Médula Ósea. German High-Grade Lymphoma Study Group. German Hodgkin's Study Group. Japanese Lymphorra Study Group. Lymphoma Study Association. NCIC Clinical Trials Group. Nordic Lymphoma Study Group. Southwest Oncology Group. United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014 Sep 20;32(27):3059-68. [PMC free article: PMC4979083] [PubMed: 25113753]
- 43.
- Kim SJ, Yoon DH, Jaccard A, Chng WJ, Lim ST, Hong H, Park Y, Chang KM, Maeda Y, Ishida F, Shin DY, Kim JS, Jeong SH, Yang DH, Jo JC, Lee GW, Choi CW, Lee WS, Chen TY, Kim K, Jung SH, Murayama T, Oki Y, Advani R, d'Amore F, Schmitz N, Suh C, Suzuki R, Kwong YL, Lin TY, Kim WS. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016 Mar;17(3):389-400. [PubMed: 26873565]
- 44.
- Au WY, Pang A, Choy C, Chim CS, Kwong YL. Quantification of circulating Epstein-Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood. 2004 Jul 01;104(1):243-9. [PubMed: 15031209]
- 45.
- Kim HS, Kim KH, Kim KH, Chang MH, Ji SH, Lim DH, Kim K, Kim SJ, Ko Y, Ki CS, Jo SJ, Lee JW, Kim WS. Whole blood Epstein-Barr virus DNA load as a diagnostic and prognostic surrogate: extranodal natural killer/T-cell lymphoma. Leuk Lymphoma. 2009 May;50(5):757-63. [PubMed: 19330658]
- 46.
- Yamaguchi M, Tobinai K, Oguchi M, Ishizuka N, Kobayashi Y, Isobe Y, Ishizawa K, Maseki N, Itoh K, Usui N, Wasada I, Kinoshita T, Ohshima K, Matsuno Y, Terauchi T, Nawano S, Ishikura S, Kagami Y, Hotta T, Oshimi K. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol. 2009 Nov 20;27(33):5594-600. [PubMed: 19805668]
- 47.
- Yamaguchi M, Tobinai K, Oguchi M, Ishizuka N, Kobayashi Y, Isobe Y, Ishizawa K, Maseki N, Itoh K, Usui N, Wasada I, Kinoshita T, Hotta T, Tsukasaki K, Oshimi K. Concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: an updated analysis of the Japan clinical oncology group study JCOG0211. J Clin Oncol. 2012 Nov 10;30(32):4044-6. [PubMed: 23045573]
- 48.
- Yamaguchi M, Suzuki R, Oguchi M, Asano N, Amaki J, Akiba T, Maeda T, Itasaka S, Kubota N, Saito Y, Kobayashi Y, Itami J, Ueda K, Miyazaki K, Ii N, Tomita N, Sekiguchi N, Takizawa J, Saito B, Murayama T, Ando T, Wada H, Hyo R, Ejima Y, Hasegawa M, Katayama N. Treatments and Outcomes of Patients With Extranodal Natural Killer/T-Cell Lymphoma Diagnosed Between 2000 and 2013: A Cooperative Study in Japan. J Clin Oncol. 2017 Jan;35(1):32-39. [PubMed: 28034070]
- 49.
- Kim SJ, Kim K, Kim BS, Kim CY, Suh C, Huh J, Lee SW, Kim JS, Cho J, Lee GW, Kang KM, Eom HS, Pyo HR, Ahn YC, Ko YH, Kim WS. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol. 2009 Dec 10;27(35):6027-32. [PubMed: 19884539]
- 50.
- Qi S, Yahalom J, Hsu M, Chelius M, Lunning M, Moskowitz A, Horwitz S. Encouraging experience in the treatment of nasal type extra-nodal NK/T-cell lymphoma in a non-Asian population. Leuk Lymphoma. 2016 Nov;57(11):2575-83. [PMC free article: PMC5592793] [PubMed: 27183991]
- 51.
- Wei W, Wu P, Li L, Zhang ZH. Effectiveness of pegaspargase, gemcitabine, and oxaliplatin (P-GEMOX) chemotherapy combined with radiotherapy in newly diagnosed, stage IE to IIE, nasal-type, extranodal natural killer/T-cell lymphoma. Hematology. 2017 Jul;22(6):320-329. [PubMed: 27917702]
- 52.
- Jing XM, Zhang ZH, Wu P, Zhang SC, Ren YR, Xiong ZJ, Wei W, Luo L, Li L. Efficacy and tolerance of pegaspargase, gemcitabine and oxaliplatin with sandwiched radiotherapy in the treatment of newly-diagnosed extranodal nature killer (NK)/T cell lymphoma. Leuk Res. 2016 Aug;47:26-31. [PubMed: 27239738]
- 53.
- Wang H, Li YX, Wang WH, Jin J, Dai JR, Wang SL, Liu YP, Song YW, Wang ZY, Liu QF, Fang H, Qi SN, Liu XF, Yu ZH. Mild toxicity and favorable prognosis of high-dose and extended involved-field intensity-modulated radiotherapy for patients with early-stage nasal NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2012 Mar 01;82(3):1115-21. [PubMed: 21514070]
- 54.
- Jaccard A, Gachard N, Marin B, Rogez S, Audrain M, Suarez F, Tilly H, Morschhauser F, Thieblemont C, Ysebaert L, Devidas A, Petit B, de Leval L, Gaulard P, Feuillard J, Bordessoule D, Hermine O., GELA and GOELAMS Intergroup. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011 Feb 10;117(6):1834-9. [PubMed: 21123825]
- 55.
- Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J, Hoekstra OS, Hicks RJ, O'Doherty MJ, Hustinx R, Biggi A, Cheson BD. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014 Sep 20;32(27):3048-58. [PMC free article: PMC5015423] [PubMed: 25113771]
- 56.
- Horwitz SM, Ansell SM, Ai WZ, Barnes J, Barta SK, Choi M, Clemens MW, Dogan A, Greer JP, Halwani A, Haverkos BM, Hoppe RT, Jacobsen E, Jagadeesh D, Kim YH, Lunning MA, Mehta A, Mehta-Shah N, Oki Y, Olsen EA, Pro B, Rajguru SA, Shanbhag S, Shustov A, Sokol L, Torka P, Wilcox R, William B, Zain J, Dwyer MA, Sundar H. NCCN Guidelines Insights: T-Cell Lymphomas, Version 2.2018. J Natl Compr Canc Netw. 2018 Feb;16(2):123-135. [PubMed: 29439173]
- 57.
- Yhim HY, Kim JS, Mun YC, Moon JH, Chae YS, Park Y, Jo JC, Kim SJ, Yoon DH, Cheong JW, Kwak JY, Lee JJ, Kim WS, Suh C, Yang DH., Consortium for Improving Survival of Lymphoma Study. Clinical Outcomes and Prognostic Factors of Up-Front Autologous Stem Cell Transplantation in Patients with Extranodal Natural Killer/T Cell Lymphoma. Biol Blood Marrow Transplant. 2015 Sep;21(9):1597-604. [PubMed: 25963920]
- 58.
- Tse E, Chan TS, Koh LP, Chng WJ, Kim WS, Tang T, Lim ST, Lie AK, Kwong YL. Allogeneic haematopoietic SCT for natural killer/T-cell lymphoma: a multicentre analysis from the Asia Lymphoma Study Group. Bone Marrow Transplant. 2014 Jul;49(7):902-6. [PubMed: 24777195]
- 59.
- Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, Khong PL, Loong F, Au-Yeung R, Iqbal J, Phipps C, Tse E. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017 Apr 27;129(17):2437-2442. [PubMed: 28188133]
- 60.
- Mei M, Wang Y, Zhang M. Causes of mortality in cases with extra nodal natural killer/T-cell lymphoma, nasal type: A cohort study. PLoS One. 2019;14(4):e0214860. [PMC free article: PMC6469770] [PubMed: 30995261]
- 61.
- Han L, Li L, Wu J, Li X, Zhang L, Wang X, Fu X, Ma W, Sun Z, Zhang X, Chang Y, Guo S, Zhang M. Clinical features and treatment of natural killer/T cell lymphoma associated with hemophagocytic syndrome: comparison with other T cell lymphoma associated with hemophagocytic syndrome. Leuk Lymphoma. 2014 Sep;55(9):2048-55. [PubMed: 24359240]
Disclosure: Aye Thida declares no relevant financial relationships with ineligible companies.
Disclosure: Pouyan Gohari declares no relevant financial relationships with ineligible companies.
- Review An Update on EBV-related Cutaneous Lymphoproliferative Disorders: a Systematic Review.[Actas Dermosifiliogr. 2024]Review An Update on EBV-related Cutaneous Lymphoproliferative Disorders: a Systematic Review.García-González S, Prieto-Torres L, García-García M. Actas Dermosifiliogr. 2024 Nov 13; . Epub 2024 Nov 13.
- Primary Nodal Epstein-Barr Virus-Positive T-Cell/NK-Cell Lymphoma: Real-World Experience.[Acta Haematol. 2024]Primary Nodal Epstein-Barr Virus-Positive T-Cell/NK-Cell Lymphoma: Real-World Experience.Choi DH, Yoon SE, Cho J, Kim SJ, Kim WS. Acta Haematol. 2024; 147(6):625-633. Epub 2024 Mar 3.
- Expression of soluble CD27 in extranodal natural killer/T-cell lymphoma, nasal type: potential as a biomarker for diagnosis and CD27/CD70-targeted therapy.[Cancer Immunol Immunother. 2023]Expression of soluble CD27 in extranodal natural killer/T-cell lymphoma, nasal type: potential as a biomarker for diagnosis and CD27/CD70-targeted therapy.Nagato T, Komatsuda H, Hayashi R, Takahara M, Kishibe K, Yasuda S, Yajima Y, Kosaka A, Ohkuri T, Oikawa K, et al. Cancer Immunol Immunother. 2023 Jul; 72(7):2087-2098. Epub 2023 Feb 22.
- Review Adenosine Deaminase Deficiency.[GeneReviews(®). 1993]Review Adenosine Deaminase Deficiency.Hershfield M, Tarrant T. GeneReviews(®). 1993
- Review Autoimmune Lymphoproliferative Syndrome.[GeneReviews(®). 1993]Review Autoimmune Lymphoproliferative Syndrome.Bleesing JJH, Nagaraj CB, Zhang K. GeneReviews(®). 1993
- Extranodal NK-Cell Lymphoma - StatPearlsExtranodal NK-Cell Lymphoma - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
See more...