NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Darifenacin is an anticholinergic and antispasmotic agent used to treat urinary incontinence and overactive bladder syndrome. Darifenacin has not been implicated in causing liver enzyme elevations or clinically apparent acute liver injury.
Background
Darifenacin (dar" i fen' a sin) is an anticholinergic agent with a degree of selectivity for the M3 subtype of muscarinic acetylcholine receptors which are found predominantly in the smooth muscle of the bladder. Darifenacin increases bladder capacity and decreases bladder contractions and the urgency of urination. Darifenacin was approved for use in the United States in 2004 and indications include urinary urge incontinence and overactive bladder syndrome. Darifenacin is available in extended release tablets of 7.5 and 15 mg generically and under the brand name Enablex. The recommended adult oral dose is 7.5 to 15 mg once daily. Common side effects are those of parasympathetic stimulation and include dryness of the mouth and eyes, decreased sweating, headache, visual blurring, constipation, and urinary retention. Because of its selectivity for the M3 muscarinic receptor, darifenacin is believed to be less likely than other anticholinergic agents to cause central nervous system effects such as restlessness, confusion and hallucinations. Rare but potentially severe adverse reactions include acute narrow angle glaucoma, acute urinary retention, and severe hypersensitivity reactions.
Hepatotoxicity
Like most anticholinergic agents, darifenacin has not been linked to liver enzyme elevations during therapy or to instances of clinically apparent liver injury with symptoms or jaundice. In multiple prospective clinical trial of darifenacin in patients with overactive bladder syndrome, ALT elevations were reported in less than 1% of treated subjects, rates similar to that of placebo recipients. Despite widespread clinical use for almost two decades, there have been no published case reports of clinically apparent liver injury convincingly attributed to darifenacin use.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Darifenacin has not been linked to liver injury. It is metabolized in the liver by microsomal P450 enzymes, predominantly CYP 3A4 and 2D6. Despite this, drug-drug interactions are uncommon. A major reason for its safety may relate to the low daily dose.
Drug Class: Anticholinergic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Darifenacin – Generic, Enablex®
DRUG CLASS
Anticholinergic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Darifenacin | 133099-04-4 | C28-H30-N2-O2 |
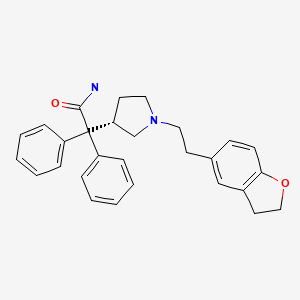
|
ANNOTATED BIBLIOGRAPHY
References updated: 12 July 2023
Abbreviations: ER, extended release.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999 before the availability of darifenacin and other therapies of overactive bladder syndrome).
- Brown JH, Brandl K, Wess J. Therapeutic uses of muscarinic receptor antagonists: Muscarinic receptor agonists and antagonists. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 156-9.(Textbook of pharmacology and therapeutics).
- Chapple C, Steers W, Norton P, Millard R, Kralidis G, Glavind K, Abrams P. A pooled analysis of three phase III studies to investigate the efficacy, tolerability and safety of darifenacin, a muscarinic M3 selective receptor antagonist, in the treatment of overactive bladder. BJU Int. 2005;95:993-1001. [PubMed: 15839920](Among 1059 adults with symptomatic overactive bladder treated with darifenacin [7.5 or 15 mg] or placebo once daily for 12 weeks in 3 controlled trials, darifenacin resulted in significant decreases in episodes of incontinence, urgency and daily micturitions compared to placebo, while adverse events arose in 54% and 66% vs 49%, the most common symptoms being dry mouth and constipation, but “there were no concerns from laboratory data”).
- Zinner N, Tuttle J, Marks L. Efficacy and tolerability of darifenacin, a muscarinic M3 selective receptor antagonist (M3 SRA), compared with oxybutynin in the treatment of patients with overactive bladder. World J Urol 2005; 23: 248-52. [PubMed: 16096831](Trial comparing darifenacin with oxybutynin in 76 patients with overactive bladder found similar rates of response but less dry mouth with darifenacin: no mention of hepatotoxicity or ALT elevations).
- Novara G, Galfano A, Secco S, D'Elia C, Cavalleri S, Ficarra V, Artibani W. A systematic review and meta-analysis of randomized controlled trials with antimuscarinic drugs for overactive bladder. Eur Urol 2008; 54: 740-63. [PubMed: 18632201](Systematic review of efficacy and safety of drugs for overactive bladder including tolterodine, propiverine, solifenacin, darifenacin, fesoterodine and oxybutynin; common side effects included dry mouth and constipation; hepatotoxicity and ALT elevations were not mentioned).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to anticholinergics or drugs for overactive bladder).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to anticholinergics or drugs for overactive bladder syndrome).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. [PMC free article: PMC2997312] [PubMed: 21039766](Among 624,673 adverse event reports in children between 2000 and 2006 in the WHO VigiBase, 1% were hepatic, but no anticholinergic was listed among the 41 most frequently implicated agents).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to mirabegron or drugs for overactive bladder syndrome).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, but none were attributed to drugs used for overactive bladder syndrome).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN Prospective Study. Gastroenterology 2015; 148: 1340-1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to mirabegron or other agents for overactive bladder syndrome).
- Drugs for overactive bladder. Med Lett Drugs Ther. 2023;65:41-45. [PubMed: 36897601](Concise review of drugs approved for therapy of overactive bladder in the US including anticholinergic agents [darifenacin, fesoterodine, oxbutynin, solifenacin, tolterodine and trospium] and beta-3 adrenergic receptor agonists [mirabegron and vibegron], including clinical efficacy, safety, and costs; no mention of ALT elevations or hepatotoxicity of any of the agent discussed).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Darifenacin - LiverToxDarifenacin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...