OVERVIEW
The aminopenicillins (sometimes referred to as third generation penicillins) are semisynthetic modifications of natural penicillin that have the advantage of a broader spectrum of activity. Like the natural penicillins, aminopenicillins have a thiazolidine ring structure connected to a beta-lactam ring which makes these agents susceptible to inactivation by beta-lactamase, the usual cause of bacterial resistance to the penicillins. The aminopenicillins, like the natural first generation penicillins, bind to bacterial proteins and inhibit synthesis of the bacterial cell wall, causing cell lysis particularly in rapidly growing organisms. The aminopenicillins are widely used for therapy of mild-to-severe urinary, respiratory, gastrointestinal tract, skin, bone and joint infections. They have activity against Escherichia coli, Hemophilus influenzae, Listeria monocytogenesis, Neisseria gonorrhoeae, Proteus mirabilis, Salmonella, Shigella, Staphylococcus aureus (non-penicillinase producing), Staphylococcus epidermidis, and Streptococcus pneumoniae.
Two third generation penicillins are available in the United States: ampicillin (am" pi sil' in) and amoxicillin (a mox" i sil' in). Both are also available in combination with a pharmacologic enhancing agent, a beta-lactamase inhibitor which extends the antibacterial coverage against penicillinase-resistant bacteria. Ampicillin is available in combination with sulbactam (sul bak' tam) sodium generically and under the trade name Unasyn. Amoxicillin is available in combination with clavulanic acid in generic forms and under the trade name Augmentin.
Bacampicillin and pivampicillin are two other aminopenicillins, but they are not available in the United States and are rarely used.
Both ampicillin and amoxicillin can cause idiosyncratic liver injury that generally resembles the injury associated with the first generation penicillins. The typical presentation is a cholestatic hepatitis arising 2 to 4 weeks after starting the antibiotic and sometimes 1 to 2 weeks after a 7- or 10-day course. The injury is usually mild-to-moderate in severity, although fatal cases of acute liver failure have been described. Importantly, with severe cholestatic hepatitis, progression to a vanishing bile duct syndrome or prolonged cholestasis can occur which rarely is fatal or leads to liver transplantation. While acute liver injury from ampicillin and amoxicillin is quite rare, liver injury from amoxicillin-clavulanate is not uncommon. In most large case series on drug induced liver injury from the United States, Europe and Australia, amoxicillin-clavulanate is far and away the most frequent cause of drug induced liver disease, while amoxicillin and ampicillin may not be listed at all. Importantly, the liver injury from amoxicillin-clavulanate is usually attributed to the clavulanate rather than the aminopenicillin, and the pattern of injury may be slightly different than that described with penicillin, ampicillin and amoxicillin alone. For these reasons, separate records are provided in LiverTox for the aminopenicillins that are used in combination with a beta-lactamase inhibitor. References to the individual agents are given with the specific drug records; general references to the aminopenicillins are given below.
Drug Class: Antiinfective Agents
The following are links to each drug record:
- Bacampicillin
- Pivampicillin
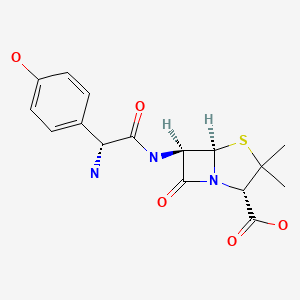
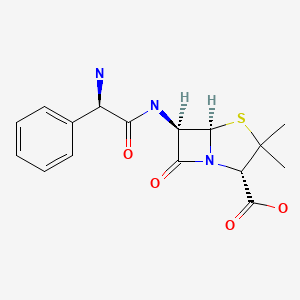
CHEMICAL FORMULAS AND STRUCTURES
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2020
- Zimmerman HJ. Penicillins. In, Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott Williams and Wilkins, 1999. p. 595-6.(Expert review of penicillins and liver injury published in 1999; the penicillins commonly lead to hypersensitivity reactions but rarely to liver injury).
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 463-82.(Review of hepatotoxicity of antibiotics mentions that penicillins rarely cause liver injury and both hepatocellular and cholestatic patterns of injury have been described).
- MacDougall C. Penicillins, cephalosporins, and other β-lactam antibiotics. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1023-38.(Textbook of pharmacology and therapeutics).
- Lees L, Milson JA, Knirsch AK, Greenhalgh K. Sulbactam plus ampicillin: interim review of efficacy and safety for therapeutic and prophylactic use. Rev Infect Dis. 1986;8 Suppl 5:S644–S650. [PubMed: 3026019](Review of 45 studies of ampicillin-sulbactam in 899 patients found successful outcome in 92%; ALT elevations in 6.9%, AST in 6.2%, but all resolved with stopping and similar rates were reported with use of comparative agents).
- Galante D, Esposito S, Barba D, Ruffilli MP. Clinical efficacy and safety of sulbactam/ampicillin in patients suffering from chronic liver disease. J Antimicrob Chemother. 1987;19:527–32. [PubMed: 3034850](41 patients with advanced liver disease were given sulbactam/ampicillin; there was no worsening of liver disease or enzyme elevations attributed to medication).
- Cavanzo FJ, Garcia CF, Botero RC. Chronic cholestasis, paucity of bile ducts, red cell aplasia, and the Stevens-Johnson syndrome. An ampicillin-associated case. Gastroenterology. 1990;99:854–6. [PubMed: 2116345](A 35 year old woman developed Stevens-Johnson syndrome with cholestatic hepatitis and red cell aplasia 4 days after starting oral ampicillin and subsequently developed vanishing bile duct syndrome and prolonged cholestasis, but with gradual ultimate improvement after several years).
- Davies MH, Harrison RF, Elias E, Hübscher SG. Antibiotic-associated acute vanishing bile duct syndrome: a pattern associated with severe, prolonged, intrahepatic cholestasis. J Hepatol. 1994;20:112–6. [PubMed: 8201211](Two cases of vanishing bile duct syndrome, one with toxic epidermolysis developing in a 37 year old woman a day after starting amoxicillin with persistent sclerosing cholangitis-like syndrome and severe jaundice documented by four liver biopsies; second case in a 42 year old woman treated with flucloxacillin who developed cholestatic hepatitis followed by persistent Alk P elevations who underwent five liver biopsies).
- Pillans PI. Drug associated hepatic reactions in New Zealand: 21 years’ experience. N Z Med J. 1996;109:315–9. [PubMed: 8816722](Adverse drug reaction reports identified 943 liver injuries over 21 years in New Zealand; amoxicillin listed in top 20 drugs over several periods).
- Friis H, Andreasen PB. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med. 1992;232:133–8. [PubMed: 1506809](Adverse drug reaction reports in Denmark from 1978 to 1987; no mention of aminopenicillins).
- Köklü S, Yüksel O, Filik L, Usküdar O, Altundag K, Altiparmak E. Recurrent cholestasis due to ampicillin. Ann Pharmacother. 2003;37:395–7. [PubMed: 12639171](23 year old man had recurrent bouts of liver injury 12, 8, and 5 days after starting three 7 day courses of ampicillin, last episode marked by bilirubin 2.1 mg/dL, ALT 265 U/L, and Alk P 455 U/L, resolving within 10 days of stopping).
- de Abajo FJ, Montero D, Madurga M, Rodriguez LAG. Acute and clinically relevant drug-induced liver injury: a population based case-control study. Brit J Clin Pharm. 2004;58:71–80. [PMC free article: PMC1884531] [PubMed: 15206996](Analysis of General Practice Research Database from UK on 1.6 million persons from 1994-2000 found 128 cases of drug induced liver injury [2.4/100,000 person-years]; amoxicillin was associated with a minimal increase in risk).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure; one case was attributed to amoxicillin-clavulanate, but none to penicillin, ampicillin or amoxicillin alone).
- Andrade RJ, Lucena MI, Kaplowitz N, Garcia-Munoz B, Borraz Y, Pachkoria K, Garcia-Cortes M, et al. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44:1581–8. [PubMed: 17133470](Clinical description of 28 patients with “chronic” outcome of drug induced liver injury included one case attributed to amoxicillin with jaundice arising 5 days after a 1 day course with severe hypersensitivity; 6 months later, only evidence of chronic injury was an elevated GGT ~1.4 times ULN).
- Björnsson E, Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatol. 2009;50:511–7. [PubMed: 19155082](Among 685 patients identified an average of 10 years after an episode of drug induced liver injury, 23 [3.4%] had continuing liver disease, 8 with cirrhosis. One patient who died of cirrhosis had ampicillin-attributed liver injury 3 years previously).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; 3 [1%] were attributed to the combination of amoxicillin and clavulanate, but none were attributed to ampicillin or amoxicillin alone].
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 66 due to antimicrobial agents, including two attributed to amoxicillin but none to ampicillin; no details given).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, amoxicillin-clavulanate accounted for 38 cases [0.4%] for an adjusted odds ratio of 1.7, whereas neither amoxicillin or ampicillin alone were listed among the 41 most common causes [linked to at least 30 cases]).
- Lucena MI, Molokhia M, Shen Y, Urban TJ, Aithal GP, Andrade RJ, Day CP, et al. Spanish DILI Registry; EUDRAGENE; DILIN; DILIGEN; International SAEC. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011 Jul;141:338–47. [PMC free article: PMC3129430] [PubMed: 21570397](A genome wide association study [GWAS] on 201 European and U.S. cases of amoxicillin-clavulanate hepatotoxicity and 532 population controls identified two strong HLA associations, one in the class II [DRB1*1501-DQB1*0602] and one in the class I region [A*0201]).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, the most commonly implicated agent being amoxicillin with clavulanate [15 cases]; none were attributed to amoxicillin or ampicillin alone).
- Ferrajolo C, Verhamme KM, Trifirò G, 't Jong GW, Giaquinto C, Picelli G, Oteri A, et al. Idiopathic acute liver injury in paediatric outpatients: incidence and signal detection in two European countries. Drug Saf. 2013;36:1007–16. [PubMed: 23591830](Analysis of 3 electronic healthcare databases from Italy and the Netherlands from 2000-2008 identified 785 cases of unexplained acute liver injury in children, linked to 110 possible medications, with increased adjusted relative risk [RR] of recent exposure to amoxicillin-clavulanate [RR=18.6] and amoxicillin [RR=7.5]).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury from Latin American countries published between 1996 and 2012 identified 176 cases, of which 37 [19%] were attributed to antimicrobials, including one to benzathine penicillin and 3 to amoxicillin-clavulanate, but none to 2nd or 4th generation penicillins).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 323 [36%] were attributed to antibiotics of which 106 [12%] were due to penicillins including one to 1st, three to 2nd [all due to oxacillin], 97 to 3rd [91 to amoxicillin-clavulanate, and 6 to amoxicillin alone] and five to 4th generation penicillins [all 5 to piperacillin/tazobactam]).
- Guéant JL, Romano A, Cornejo-Garcia JA, Oussalah A, Chery C, Blanca-López N, Guéant-Rodriguez RM, et al. HLA-DRA variants predict penicillin allergy in genome-wide fine-mapping genotyping. J Allergy Clin Immunol. 2015;135:253–9. [PubMed: 25224099](In a genome wide association study of 387 patients with immediate allergic reactions to beta-lactam antibiotics, several class 2 HLA associations [HLA-DRA regions] were found for penicillin responses, but they did not apply to cephalosporin cases).
- Nicoletti P, Aithal GP, Björnsson ES, Andrade RJ, Sawle A, Arrese M, Barnhart HX, et al. International Drug-Induced Liver Injury Consortium, Drug-Induced Liver Injury Network Investigators, and International Serious Adverse Events Consortium. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology. 2017;152:1078–89. [PMC free article: PMC5367948] [PubMed: 28043905](A genome wide association study done on 862 Caucasian patients with drug induced liver injury demonstrated a strong link with HLA-A*33:01 in those with cholestatic liver injury, particularly in cases attributed to terbinafine, fenofibrate and ticlopidine).
- Overby CL, Hripcsak G, Shen Y. Estimating heritability of drug-induced liver injury from common variants and implications for future study designs. Sci Rep. 2014;4:5762. [PMC free article: PMC4104390] [PubMed: 25042059](Genome wide complex trait analysis suggests that most of the heritability of flucloxacillin induced liver injury is attributable to findings on chromosome 6 [HLA associations] as contrasted to amoxicillin-clavulanate for which chromosome six single nucleotide polymorphisms account for less than half the heritability).
- Tailor A, Faulkner L, Naisbitt DJ, Park BK. The chemical, genetic and immunological basis of idiosyncratic drug-induced liver injury. Hum Exp Toxicol. 2015;34:1310–7. [PubMed: 26614821](Review of mechanisms of idiosyncratic drug induced liver injury focusing upon chemically reactive drug metabolites and genetic associations, particularly those with HLA alleles that implicate the adaptive immune response).
- Cirulli ET, Nicoletti P, Abramson K, Andrade RJ, Bjornsson ES, Chalasani N, Fontana RJ, et al. Drug-Induced Liver Injury Network (DILIN) investigators. International DILI consortium (iDILIC). A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology. 2019;156:1707–16.e2. [PMC free article: PMC6511989] [PubMed: 30664875](Genome wide association studies on 2048 patients with drug induced liver injury and 12,439 controls identified a variant in PTPN22 which was highly associated with liver injury, allele frequency being 0.12 among cases and 0.08 among controls with highest association in Northern Europeans and in cases of amoxicillin clavulanate, PTPN22 being a cellular kinase involved in modulation of immune reactions).
Publication Details
Publication History
Last Update: October 20, 2020.
Copyright
Publisher
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda (MD)
NLM Citation
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Penicillins (3rd Generation) [Updated 2020 Oct 20].