This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-.

StatPearls [Internet].
Show detailsContinuing Education Activity
Radiation-induced coronary artery disease (RICAD) is one of the most common causes of morbidity and mortality in patients treated with chest radiation therapy (RT) for cancer. With increased rates of oncologic treatment, the rates of RICAD are increasing in parallel. This activity will discuss in detail the etiology of RICAD, epidemiology, clinical presentation, evaluation, screening recommendations, treatment options, differential diagnosis, and complications, as well as the role of the interprofessional team.
Objectives:
- Describe the pathophysiology of radiation-induced coronary artery disease.
- Review the presentation of a patient with radiation-induced coronary artery disease.
- Summarize the surveillance and treatment options for radiation-induced coronary artery disease.
- Review the importance of improving care coordination among interprofessional team members to improve outcomes for patients affected by radiation-induced coronary artery disease.
Introduction
Radiation-induced coronary artery disease (RICAD) is a known cause of morbidity and mortality in patients treated with chest radiation therapy (RT) for cancer but has also been known to occur from other forms of radiation exposure.[1] With the increased use of RT as part of cancer therapy, the rates of RICAD have been increasing in parallel.[2] It is thought to occur due to accelerated atherosclerosis of the coronary vasculature.[3]
RICAD affects younger patients, is closely associated with the type of chest RT and radiation dose, and usually affects proximal epicardial coronaries, including left main arterial disease.[4] This article will discuss in detail the etiology of RICAD, epidemiology, clinical presentation, evaluation, screening recommendations, treatment options, differential diagnosis, and complications.
Etiology
Radiation exposure can be natural or iatrogenic. Most natural exposure does not result in high radiation levels at a single time, and sources of this type of exposure include radon gas in the ground seeping into basements and crawlspaces, cosmic rays from sun exposure, and high altitude.[2] More often, patients are exposed to radiation through medical imaging or therapy, which explains the high incidence of RICAD in post-RT patients. Radiation-induced coronary artery disease is primarily caused by prior mediastinal radiation exposure for multiple cancers affecting the mediastinum, including breast cancer, Hodgkin’s lymphoma, lung cancer, esophageal cancer, and thymoma.[5]
The degree and incidence of RICAD are closely associated with the location and dose of RT delivered and the total number of fractions, with patients receiving chest radiation having a prolonged risk of RICAD and associated myocardial infarction up to 25 years after initial RT.[6] A mean mediastinal radiation dose greater than 30 Gy was associated with an increased risk of death from cardiac causes.[7] A prior study on breast cancer patients receiving RT suggests that the risk of major cardiac events was noted to increase by 7.4% per Gy increase in the mean heart dose of radiation.[8] In addition to the RT dose itself, there are other known risk factors for RICAD that include age at exposure (younger patients at higher risk), concomitant use of cardiotoxic drugs like anthracyclines as well as known risk factors for coronary artery disease (CAD) including smoking, diabetes, hyperlipidemia, hypertension, and obesity.[9]
Epidemiology
The most common conditions associated with RICAD are breast cancer (BC) and Hodgkin lymphoma (HL).[5] RT for lung cancer, esophageal cancer, and thymoma are also less commonly known to cause RICAD.[10] HL survivors treated with radiation therapy have a 2.5 times higher risk of CAD, whereas BC patients only have 0.25 times higher risk.[8][11][12] This is because patients with HL receive a higher dose of RT than patients with BC, which represents a dose-dependent effect on the development of RICAD.[13] The reported absolute excess risk of myocardial infarction in patients with HL who received chest radiation is 125.8 per 100,000 person-years.[6] Similarly, the reported risk increase of cardiac death in BC patients who received chest RT is 125.5 per 100,000 years.[14] Even though the incidence of CAD in BC patients who receive RT is lower, it is known to increase linearly with time.[15]
Younger age at the time of RT exposure is associated with increased risk for RICAD, with a study on patients receiving RT for HL showing the highest relative risk of RICAD in patients under 25 years of age at the time of exposure.[16] Initially, the incidence of RICAD following left-sided RT was higher than right-sided RT. However, more recently, there have been reports of higher rates of RICAD following right-sided RT, specifically involving the right coronary artery.[17][18]
Pathophysiology
The pathophysiology of RICAD is theorized to be a result of accelerated atherosclerotic disease of the coronaries.[3] There is reportedly both microvascular and macrovascular endothelial damage in coronary arteries.[19] Radiation particles (ions) cause the initial cellular injury initiating a pro-inflammatory state. The NF-kB pathway has been considered one factor leading to long-term oxidative stress.[13] This endothelial injury not only leads to epicardial and microvascular damage but also causes vasoconstriction. Pro-thrombotic factors like thrombomodulin combine with endothelial injury and lead to a pro-thrombotic vasoconstrictive state with hemostasis imbalance. Over time, fibrosis ensues both at the microvascular and macrovascular level and promotes plaque formation in the epicardial coronaries.[20] The resulting occlusion and/or compromised coronary arterial blood flow has been known to evolve into myocardial ischemia and infarction.[3]
Histopathology
The coronary arteries are particularly vulnerable to RT of the chest. These arteries consist of three wall layers (tunica intima, media, and adventitia), of which only the tunica intima comprises the endothelium.[21] Endothelial cells respond to RT in a variety of ways. Depending on the radiation dose, cells can develop a range of problems, from DNA breakage to apoptosis.[22] On a histologic level, RT results in a variety of tissue damage, such as cellular loss, an influx of inflammatory cells and markers, and fibrin plaques in advanced disease-causing partial or complete occlusion. Atherosclerotic plaques in RICAD are different in that there is a greater degree of fibrosis and the lesions appear longer with a lower lipid burden and more significant hyperplasia of the intima.[23]
An autopsy study of HL patients who had received RT during their lifetimes noted that atherosclerosis was disproportionate to expected based on age and risk factors. The most commonly involved vessels were the proximal epicardial coronaries, with left main and right ostial lesions being the most common. Additionally, there was no evidence of small vessel disease or vasculitis of coronaries.[13][24]
History and Physical
The classic presentation of RICAD would be CAD in a young patient who has previously been exposed to thoracic RT with or without traditional risk factors for CAD.[25] Patients have been known to present across a wide spectrum with either typical anginal symptoms, atypical angina, or even silent myocardial ischemic/infarction.[26] Similar to silent myocardial infarction in patients with diabetes, autonomic dysfunction post-RT has been demonstrated and may be the cause of atypical or fewer symptoms in patients with RICAD.[4] Sudden cardiac death is also a less common presentation of RICAD. This is thought to be from either left main stenosis or proximal vessel ostial obstructive CAD.[27]
There may be decades between initial exposure to RT and symptoms of RICAD. The mean time from radiation exposure to the development of RICAD is estimated at 82 months, and patients are usually less than 50 years old at the time of presentation although cases have been reported in children and adolescents as well.[24][28]
Physical examination findings in RICAD are similar to patients with CAD in the general population. A study on patients who received RT for HL showed abnormal resting heart rate and abnormal heart rate recovery post-exercise.[4] Patients with RICAD may have dyspnea, lower extremity edema, bi-basilar pulmonary crackles, and jugular venous distension due to the development of ischemic cardiomyopathy. However, there is a higher incidence of diastolic dysfunction than systolic dysfunction in these patients.[29] RICAD is also associated with a higher incidence of valvular disease, and a cardiovascular exam may demonstrate heart murmurs.[30]
Evaluation
One of the keys to evaluating RICAD is the surveillance of patients with a known history of chest RT. The European Society of Cardiology recommends an evaluation for CAD starting as early as ten years post thoracic RT, with continued surveillance testing every five years, regardless of symptoms.[31] Although there is no clear data on whether or not asymptomatic patients with evidence of obstructive RICAD should be preferentially managed with medical therapy versus undergoing revascularization.
Biomarkers like brain natriuretic peptides and troponin have been shown to be elevated in patients with RICAD.[32] In addition, high sensitivity C reactive protein has been found to increase on serial measurements in patients with evidence of RICAD.[33] Coronary artery calcium on non-contrast chest CT has demonstrated high Agatston scores in patients with RICAD. Furthermore, a coronary calcium score of 0 was noted to have an excellent negative predictive value in the HL patients with a history of RT.[11]
Functional stress testing for CAD has been studied using stress echocardiography and stress myocardial perfusion imaging. There is a high incidence of perfusion defects suggesting ischemia noted.[32] However, evidence suggests this does not correlate well with hemodynamically significant epicardial coronary artery disease as often, which may be due to significant coronary microvascular disease. On the other hand, a study on anatomic testing with coronary CT angiography demonstrated an increase in coronary lesions over ten years in post-chest RT patients with a history of HL. The correlation with angiography for severe lesions was more consistent with only a small fraction of patients requiring revascularization.[34]
The most commonly involved vessel in patients who received RT for BC is left main disease due to left-sided radiation. In patients with HL, all proximal vessels, including the right coronary artery, left main, left anterior descending artery, and left circumflex artery, can be involved, with some data suggesting the right coronary is most commonly involved.[31] This is attributed to the anterior position of these vessels resulting in higher radiation exposure.
For patients who present with symptoms concerning for acute coronary syndrome, the evaluation should be based on clinical risk score-based decision pathways used for CAD in the general population. High-risk patients should undergo invasive coronary angiography, and intermediate-risk patients should undergo either anatomic or functional testing for CAD.[31]
Treatment / Management
In patients with a diagnosis of RICAD, aspirin and statins are reasonable to prevent major adverse cardiovascular events. In animal models, captopril and simvastatin decreased the effects of a radiation-induced injury.[35][36][35] There is also evidence to support colchicine as prophylactic therapy for RICAD.[37] However, the use of these agents has not been studied in prospective human trials, and there are no guidelines that outline the use of prophylactic medication to prevent the development of RICAD.
In terms of revascularization therapy, percutaneous coronary intervention has been widely used to manage RICAD in the same way as patients with CAD in the general population. Earlier studies that looked at patients who received bare-metal stents for obstructive RICAD showed high rates of target lesion revascularization, thought to be due to accelerated atherosclerosis.[38] However, with the advent of drug-eluting stents, this need for repeat revascularization has significantly dropped.[39]
Revascularization with coronary artery bypass surgery is fraught with multiple obstacles. Firstly, mediastinal RT causes not just coronary artery fibrosis but affects other structures in the anterior mediastinum, including the chest wall, pericardium, and lung tissue, which may also be affected by radiation-induced fibrosis. Postoperative pulmonary complications are much more common as a result.[40] Secondly, in patients with prior chest RT, there is a higher need for reoperations and compound procedures at the time of bypass grafting, like one or more valve replacements, since these patients have a higher rate of valvular dysfunction.[41][42] Thirdly, the degree of fibrosis and prior damage to the chest wall often leads to poor wound healing after bypass surgery.[32]
Lastly, there is evidence that in these patients, internal mammary grafts do not remain patent as long as in the general population due to having received radiation exposure as well, making conduit selection for bypass grafting very challenging.[43]
Differential Diagnosis
The differential diagnosis for RICAD includes conditions that can mimic stable or unstable CAD and acute coronary syndrome. It also includes other conditions that are associated with thoracic radiation.
- Pericarditis (acute and chronic constrictive)
- Pulmonary embolism
- Coronary microvascular dysfunction
- Restrictive cardiomyopathy
- Pulmonary fibrosis
- Costochondritis
- Kawasaki disease
- Myocarditis
- Esophagitis
Prognosis
Besides the inherent increased morbidity and mortality in patients with RICAD at a younger age than CAD patients in the general population, certain risk factors portend a poorer prognosis in these patients. In patients with pre-existing CAD, the risk of adverse cardiovascular events increases by 60% following RT.[44] A sedentary lifestyle has also been associated with an increased risk of cardiac events in patients with a history of chest RT for HL or BC.[32]
Complications
Complications of RICAD are similar to CAD in the general population, including:
- Acute coronary syndrome
- Ischemic cardiomyopathy
- Acute heart failure
- Arrhythmias
- Valvular dysfunction
- Sudden cardiac death[26]
Deterrence and Patient Education
Patient education starts with the initial discussion before the initiation of RT. Patients should be informed of their risk for developing RICAD based on the expected radiation dose, concomitant chemotherapy, and other risk factors. Other options for treatment should be offered, and if RT is considered the best option, patients should be referred to centers specializing in focused RT, like photon therapy. Patients who undergo RT should discuss the role of surveillance imaging and overall CAD risk reduction with their providers.
Pearls and Other Issues
Prevention
3D conformal radiotherapy, proton therapy, and other new forms of delivering RT that focus the radiation beam away from the heart should be used to cut down the cumulative radiation dose to the heart.[26] In addition, breath-holding techniques can also be used for RT of left-sided breast cancer, which moves the breast away from the heart during therapy.[45] The traditionally used atherosclerotic cardiovascular disease – ASCVD risk calculator does not take into account a history of chest RT.[46]
However, given the inherently increased risk of CAD with chest radiation, patients with a history of the same should undergo aggressive CAD risk factor modification, including smoking cessation, diabetes, and hyperlipidemia management. [26] There is currently no role for prophylactic treatment of patients with a history of chest RT with statins or aspirin to prevent RICAD, although study data in this regard is sparse.[47] Small studies have found that statins can reduce radiation-induced tissue fibrosis and oxidative damage to DNA in the vasculature; however, randomized clinical trial data are lacking.[48]
Enhancing Healthcare Team Outcomes
Radiation-induced coronary artery disease may be overlooked as a potential diagnosis. An interprofessional team approach can sometimes aid in narrowing the diagnosis. Members should include but are not limited to the primary care providers (including MDs, DOs, NPs, and PAs), cardiologists, oncologists, radiation oncologists, nursing staff, and pharmacists. Emergency room providers and nurses should be trained in recognizing the risk of RICAD in patients with a history of prior thoracic radiation, especially given its presentation in younger patients. A multidisciplinary clinical approach combined with an interprofessional team approach with a high index of suspicion with a concerning history is likely to significantly increase early recognition of this condition by providers and optimize healthcare outcomes.
Review Questions

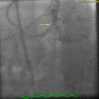
Figure
Coronary angiogram showing severe left main coronary artery disease with a bifurcation lesion involving the ostial left anterior descending artery and the ostial left circumflex artery. Contributed by Kifah Hussain MD
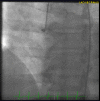
Figure
Coronary angiogram in a patient with radiation-induced coronary artery disease with blunt stump chronic total occlusion of the right coronary artery. Contributed by Kifah Hussain, MD
References
- 1.
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011 Nov 12;378(9804):1707-16. [PMC free article: PMC3254252] [PubMed: 22019144]
- 2.
- Raghunathan D, Khilji MI, Hassan SA, Yusuf SW. Radiation-Induced Cardiovascular Disease. Curr Atheroscler Rep. 2017 May;19(5):22. [PubMed: 28315200]
- 3.
- da Silva RMFL. Effects of Radiotherapy in Coronary Artery Disease. Curr Atheroscler Rep. 2019 Nov 19;21(12):50. [PubMed: 31741087]
- 4.
- Groarke JD, Tanguturi VK, Hainer J, Klein J, Moslehi JJ, Ng A, Forman DE, Di Carli MF, Nohria A. Abnormal exercise response in long-term survivors of hodgkin lymphoma treated with thoracic irradiation: evidence of cardiac autonomic dysfunction and impact on outcomes. J Am Coll Cardiol. 2015 Feb 17;65(6):573-83. [PubMed: 25677317]
- 5.
- Filopei J, Frishman W. Radiation-induced heart disease. Cardiol Rev. 2012 Jul-Aug;20(4):184-8. [PubMed: 22314140]
- 6.
- Swerdlow AJ, Higgins CD, Smith P, Cunningham D, Hancock BW, Horwich A, Hoskin PJ, Lister A, Radford JA, Rohatiner AZ, Linch DC. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst. 2007 Feb 07;99(3):206-14. [PubMed: 17284715]
- 7.
- Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA. 1993 Oct 27;270(16):1949-55. [PubMed: 8411552]
- 8.
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013 Mar 14;368(11):987-98. [PubMed: 23484825]
- 9.
- Om A, Ellahham S, Vetrovec GW. Radiation-induced coronary artery disease. Am Heart J. 1992 Dec;124(6):1598-602. [PubMed: 1462919]
- 10.
- Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A, Kavadi V, Garces YI, Narayan S, Iyengar P, Robinson C, Wynn RB, Koprowski C, Meng J, Beitler J, Gaur R, Curran W, Choy H. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015 Feb;16(2):187-99. [PMC free article: PMC4419359] [PubMed: 25601342]
- 11.
- Andersen R, Wethal T, Günther A, Fosså A, Edvardsen T, Fosså SD, Kjekshus J. Relation of coronary artery calcium score to premature coronary artery disease in survivors >15 years of Hodgkin's lymphoma. Am J Cardiol. 2010 Jan 15;105(2):149-52. [PubMed: 20102909]
- 12.
- van Nimwegen FA, Schaapveld M, Cutter DJ, Janus CP, Krol AD, Hauptmann M, Kooijman K, Roesink J, van der Maazen R, Darby SC, Aleman BM, van Leeuwen FE. Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J Clin Oncol. 2016 Jan 20;34(3):235-43. [PubMed: 26573075]
- 13.
- DeZorzi C. Radiation-Induced Coronary Artery Disease and Its Treatment: A Quick Review of Current Evidence. Cardiol Res Pract. 2018;2018:8367268. [PMC free article: PMC6206518] [PubMed: 30410795]
- 14.
- Cheng YJ, Nie XY, Ji CC, Lin XX, Liu LJ, Chen XM, Yao H, Wu SH. Long-Term Cardiovascular Risk After Radiotherapy in Women With Breast Cancer. J Am Heart Assoc. 2017 May 21;6(5) [PMC free article: PMC5524103] [PubMed: 28529208]
- 15.
- Takx RAP, Vliegenthart R, Schoepf UJ, Pilz LR, Schoenberg SO, Morris PB, Henzler T, Apfaltrer P. Coronary artery calcium in breast cancer survivors after radiation therapy. Int J Cardiovasc Imaging. 2017 Sep;33(9):1425-1431. [PubMed: 28342038]
- 16.
- van Nimwegen FA, Schaapveld M, Janus CP, Krol AD, Petersen EJ, Raemaekers JM, Kok WE, Aleman BM, van Leeuwen FE. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015 Jun;175(6):1007-17. [PubMed: 25915855]
- 17.
- Rehammar JC, Jensen MB, McGale P, Lorenzen EL, Taylor C, Darby SC, Videbæk L, Wang Z, Ewertz M. Risk of heart disease in relation to radiotherapy and chemotherapy with anthracyclines among 19,464 breast cancer patients in Denmark, 1977-2005. Radiother Oncol. 2017 May;123(2):299-305. [PMC free article: PMC5446317] [PubMed: 28365142]
- 18.
- Altınok A, Askeroğlu O, Doyuran M, Çağlar M, Cantürk E, Erol C, Beşe N. Dosimetric evaluation of right coronary artery in radiotherapy for breast cancer. 2019 AutumnMed Dosim. 44(3):205-209. [PubMed: 30170990]
- 19.
- Venkatesulu BP, Mahadevan LS, Aliru ML, Yang X, Bodd MH, Singh PK, Yusuf SW, Abe JI, Krishnan S. Radiation-Induced Endothelial Vascular Injury: A Review of Possible Mechanisms. JACC Basic Transl Sci. 2018 Aug;3(4):563-572. [PMC free article: PMC6115704] [PubMed: 30175280]
- 20.
- Armanious MA, Mohammadi H, Khodor S, Oliver DE, Johnstone PA, Fradley MG. Cardiovascular effects of radiation therapy. Curr Probl Cancer. 2018 Jul;42(4):433-442. [PubMed: 30006103]
- 21.
- Virmani R, Farb A, Carter AJ, Jones RM. Comparative pathology: radiation-induced coronary artery disease in man and animals. Semin Interv Cardiol. 1998 Sep-Dec;3(3-4):163-72. [PubMed: 10406688]
- 22.
- Rak J, Chomicz L, Wiczk J, Westphal K, Zdrowowicz M, Wityk P, Żyndul M, Makurat S, Golon Ł. Mechanisms of Damage to DNA Labeled with Electrophilic Nucleobases Induced by Ionizing or UV Radiation. J Phys Chem B. 2015 Jul 02;119(26):8227-38. [PubMed: 26061614]
- 23.
- Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013 Jun 11;61(23):2319-28. [PubMed: 23583253]
- 24.
- Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996 Aug;27(8):766-73. [PubMed: 8760008]
- 25.
- Ruiz CR, Mesa-Pabón M, Soto K, Román JH, López-Candales A. Radiation-Induced Coronary Artery Disease in Young Patients. Heart Views. 2018 Jan-Mar;19(1):23-26. [PMC free article: PMC5965011] [PubMed: 29876028]
- 26.
- Kirresh A, White L, Mitchell A, Ahmad S, Obika B, Davis S, Ahmad M, Candilio L. Radiation-induced coronary artery disease: a difficult clinical conundrum. Clin Med (Lond). 2022 May;22(3):251-256. [PMC free article: PMC9135079] [PubMed: 35584837]
- 27.
- Brosius FC, Waller BF, Roberts WC. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med. 1981 Mar;70(3):519-30. [PubMed: 6782873]
- 28.
- McEniery PT, Dorosti K, Schiavone WA, Pedrick TJ, Sheldon WC. Clinical and angiographic features of coronary artery disease after chest irradiation. Am J Cardiol. 1987 Nov 01;60(13):1020-4. [PubMed: 3673902]
- 29.
- Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005 Nov;150(5):977-82. [PubMed: 16290974]
- 30.
- Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, Greenbaum N, Mauch P, Lipshultz SE. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004 Aug 01;22(15):3139-48. [PubMed: 15284266]
- 31.
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM., ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016 Sep 21;37(36):2768-2801. [PubMed: 27567406]
- 32.
- Cuomo JR, Javaheri SP, Sharma GK, Kapoor D, Berman AE, Weintraub NL. How to prevent and manage radiation-induced coronary artery disease. Heart. 2018 Oct;104(20):1647-1653. [PMC free article: PMC6381836] [PubMed: 29764968]
- 33.
- Chen MH, Blackington LH, Zhou J, Chu TF, Gauvreau K, Marcus KJ, Fisher DC, Diller LR, Ng AK. Blood pressure is associated with occult cardiovascular disease in prospectively studied Hodgkin lymphoma survivors after chest radiation. Leuk Lymphoma. 2014 Nov;55(11):2477-83. [PubMed: 24397615]
- 34.
- Girinsky T, M'Kacher R, Lessard N, Koscielny S, Elfassy E, Raoux F, Carde P, Santos MD, Margainaud JP, Sabatier L, Ghalibafian M, Paul JF. Prospective coronary heart disease screening in asymptomatic Hodgkin lymphoma patients using coronary computed tomography angiography: results and risk factor analysis. Int J Radiat Oncol Biol Phys. 2014 May 01;89(1):59-66. [PubMed: 24613809]
- 35.
- Wei J, Xu H, Liu Y, Li B, Zhou F. Effect of captopril on radiation-induced TGF-β1 secretion in EA.Hy926 human umbilical vein endothelial cells. Oncotarget. 2017 Mar 28;8(13):20842-20850. [PMC free article: PMC5400550] [PubMed: 28209920]
- 36.
- Wilkinson EL, Sidaway JE, Cross MJ. Statin regulated ERK5 stimulates tight junction formation and reduces permeability in human cardiac endothelial cells. J Cell Physiol. 2018 Jan;233(1):186-200. [PMC free article: PMC5655747] [PubMed: 28639275]
- 37.
- O'Herron T, Lafferty J. Prophylactic use of colchicine in preventing radiation induced coronary artery disease. Med Hypotheses. 2018 Feb;111:58-60. [PubMed: 29406998]
- 38.
- Schömig K, Ndrepepa G, Mehilli J, Pache J, Kastrati A, Schömig A. Thoracic radiotherapy in patients with lymphoma and restenosis after coronary stent placement. Catheter Cardiovasc Interv. 2007 Sep;70(3):359-65. [PubMed: 17722039]
- 39.
- Reed GW, Masri A, Griffin BP, Kapadia SR, Ellis SG, Desai MY. Long-Term Mortality in Patients With Radiation-Associated Coronary Artery Disease Treated With Percutaneous Coronary Intervention. Circ Cardiovasc Interv. 2016 Jun;9(6) [PubMed: 27313281]
- 40.
- Donnellan E, Phelan D, McCarthy CP, Collier P, Desai M, Griffin B. Radiation-induced heart disease: A practical guide to diagnosis and management. Cleve Clin J Med. 2016 Dec;83(12):914-922. [PubMed: 27938516]
- 41.
- Dolmaci OB, Farag ES, Boekholdt SM, van Boven WJP, Kaya A. Outcomes of cardiac surgery after mediastinal radiation therapy: A single-center experience. J Card Surg. 2020 Mar;35(3):612-619. [PMC free article: PMC7079019] [PubMed: 31971292]
- 42.
- Handa N, McGregor CG, Danielson GK, Orszulak TA, Mullany CJ, Daly RC, Dearani JA, Anderson BJ, Puga FJ. Coronary artery bypass grafting in patients with previous mediastinal radiation therapy. J Thorac Cardiovasc Surg. 1999 Jun;117(6):1136-42. [PubMed: 10343262]
- 43.
- Brown ML, Schaff HV, Sundt TM. Conduit choice for coronary artery bypass grafting after mediastinal radiation. J Thorac Cardiovasc Surg. 2008 Nov;136(5):1167-71. [PubMed: 19026798]
- 44.
- McGale P, Darby SC, Hall P, Adolfsson J, Bengtsson NO, Bennet AM, Fornander T, Gigante B, Jensen MB, Peto R, Rahimi K, Taylor CW, Ewertz M. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011 Aug;100(2):167-75. [PubMed: 21752480]
- 45.
- Sripathi LK, Ahlawat P, Simson DK, Khadanga CR, Kamarsu L, Surana SK, Arasu K, Singh H. Cardiac Dose Reduction with Deep-Inspiratory Breath Hold Technique of Radiotherapy for Left-Sided Breast Cancer. J Med Phys. 2017 Jul-Sep;42(3):123-127. [PMC free article: PMC5618457] [PubMed: 28974856]
- 46.
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Jun 18;139(25):e1082-e1143. [PMC free article: PMC7403606] [PubMed: 30586774]
- 47.
- Camara Planek MI, Silver AJ, Volgman AS, Okwuosa TM. Exploratory Review of the Role of Statins, Colchicine, and Aspirin for the Prevention of Radiation-Associated Cardiovascular Disease and Mortality. J Am Heart Assoc. 2020 Jan 21;9(2):e014668. [PMC free article: PMC7033839] [PubMed: 31960749]
- 48.
- Wang H, Wei J, Zheng Q, Meng L, Xin Y, Yin X, Jiang X. Radiation-induced heart disease: a review of classification, mechanism and prevention. Int J Biol Sci. 2019;15(10):2128-2138. [PMC free article: PMC6775290] [PubMed: 31592122]
Disclosure: Kristen Brown declares no relevant financial relationships with ineligible companies.
Disclosure: Kifah Hussain declares no relevant financial relationships with ineligible companies.
Disclosure: John Richards declares no relevant financial relationships with ineligible companies.
- Continuing Education Activity
- Introduction
- Etiology
- Epidemiology
- Pathophysiology
- Histopathology
- History and Physical
- Evaluation
- Treatment / Management
- Differential Diagnosis
- Prognosis
- Complications
- Deterrence and Patient Education
- Pearls and Other Issues
- Enhancing Healthcare Team Outcomes
- Review Questions
- References
- Gallium Scan.[StatPearls. 2025]Gallium Scan.Dittrich RP, De Jesus O. StatPearls. 2025 Jan
- RETRACTED: Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial.[Int J Antimicrob Agents. 2020]RETRACTED: Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, et al. Int J Antimicrob Agents. 2020 Jul; 56(1):105949. Epub 2020 Mar 20.
- Symptomatic Carotid Artery Stenosis.[StatPearls. 2025]Symptomatic Carotid Artery Stenosis.Qaja E, Tadi P, Theetha Kariyanna P. StatPearls. 2025 Jan
- Review Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.[The Time of Anthropology: Stud...]Review Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.Salisbury L, Baraitser L. The Time of Anthropology: Studies of Contemporary Chronopolitics. 2020
- Review Retinoblastoma.[GeneReviews(®). 1993]Review Retinoblastoma.Lohmann DR, Gallie BL. GeneReviews(®). 1993
- Radiation-Induced Coronary Artery Disease - StatPearlsRadiation-Induced Coronary Artery Disease - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
See more...