NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Cancer (UK). Neutropenic Sepsis: Prevention and Management of Neutropenic Sepsis in Cancer Patients. London: National Institute for Health and Clinical Excellence (NICE); 2012 Sep. (NICE Clinical Guidelines, No. 151.)
This chapter provides a summary of the needs assessment that was carried out to inform development of this guideline and includes current information available regarding the epidemiology of neutropenic sepsis and existing service provision across England and Wales. The full needs assessment report can be found as a supplementary document accompanying the guideline.
1.1. Introduction
The purpose of this guideline is to ensure prompt and effective management of cancer patients presenting with neutropenic sepsis, as well as advising on prevention and diagnosis of this important complication of anticancer treatments. It is a significant cause of mortality and morbidity and causes delays and dose reductions to planned treatment. The greatest risk of neutropenic sepsis is with cytotoxic chemotherapy. The Guideline Development Group (GDG) recognises the importance of distinguishing uncomplicated neutropenic fever from neutropenia with severe sepsis and shock, and indeed septic shock can occur without fever. In clinical practice the terms febrile neutropenia and neutropenic sepsis are used interchangeably in this patient group and recommendations within this guideline use the term “neutropenic sepsis” to indicate the full range of severity of illness.
The neutrophils or granulocytes form part of the innate immune system. Normally they constitute 60-70% of the total leukocyte count. They circulate in the blood and are found inactive in the bone marrow. Neutrophils respond early to signals reporting injury or infection, migrating to the affected area. They have a role in both directly killing non-host cells such as bacteria by phagocytosis and chemical damage via degranulation, and activating other parts of the immune system, for example T cells (Nathan, 2006, Witko-Sarsat, et al., 2000). They have a circulating life span of between 8 hours and 5 days (Pillay, et al., 2010), and take approximately six days to enter circulation from the bone marrow (Dancey, et al., 1976).
Cytotoxic anticancer chemotherapy is designed to kill neoplastic stem cells by damaging the DNA irreparably. The mechanism behind this damage varies according to the chemotherapy drug. The more rapidly dividing normal cells such as hair follicles, mucosal linings and bone marrow cells can also be affected, causing the well documented toxicities of alopecia, mucositis and bone marrow suppression leading to neutropenia, anaemia and thrombocytopenia. For the majority of chemotherapy regimens, the neutrophil count falls to its lowest level approximately 5-7 days after administration of chemotherapy (Holmes, 2002), and can take up to 2-4 weeks to recover, although for some drugs and regimens, these timescales are considerably different. There is a tendency for neutropenic sepsis to occur more commonly in the first two cycles of treatment (Lyman and Delgado, 2003). While novel biological agents generally have a lower rate of neutropenia than cytotoxic chemotherapy, such problems can still occur.
When neutropenic, the patient is vulnerable to invasive infection (Bhatt and Saleem, 2004) which can potentially cause overwhelming sepsis and death. Deterioration can be very rapid, sometimes without an obvious focus for infection. Reported mortality for untreated neutropenic sepsis ranges from 2 to 21% (Herbst, et al., 2009). Neutropenic sepsis is therefore considered a medical emergency, and as with severe sepsis and septic shock from any cause, there is widespread agreement that early administration of broad spectrum antibiotics and management of shock is key to successful treatment (Rivers, et al., 2001). There is almost no universal agreement about the details of many aspects of the care of a patient with neutropenic sepsis, although there are many common themes (Phillips, et al., 2007).
There are various strategies for preventing neutropenic sepsis. Primary prophylaxis aims to prevent first episodes of neutropenic sepsis, and secondary prophylaxis is a strategy used to prevent subsequent episodes. Granulocyte colony stimulating factors (GCSF), antibiotics, and alterations to the cytotoxic regimen are the main prophylactic strategies.
Recently neutropenic sepsis has been highlighted as an area of clinical priority in the UK, initially by a publication from the National Confidential Enquiry into Patient Outcome and Death (NCEPOD 2008) then by a subsequent report from the National Chemotherapy Advisory Group (NCAG, 2009).
In 2008, NCEPOD published “For better or for worse? A review of the care of patients who died within 30 days of receiving anticancer therapy” (NCEPOD, 2008). This report looked at the deaths of patients within 30 days of chemotherapy, and highlighted aspects of care which could be improved. Recommendations covered the development of appropriate clinical care pathways and local policies, staff training and timely availability of antibiotics. A specific recommendation was made for antibiotics to be given within 30 minutes of presentation to patients with suspected neutropenic sepsis and shock.
Following the NCEPOD report, (NCEPOD, 2008) NCAG published “Chemotherapy Services in England: Ensuring quality and safety” (NCAG, 2009). The aim of the report was “to bring about a step change in the quality and safety of chemotherapy services in England, taking account of the concerns from peer review and from NCEPOD”. Key recommendations made included, the introduction of acute oncology provision, appropriate patient education and access to emergency advice and healthcare. A “door to needle” time of one hour was recommended for antibiotics to be administered in cases of suspected neutropenic sepsis.
Current practice concerning the management of neutropenic sepsis has also been influenced by many other international recommendations, guidelines and studies.
The Surviving Sepsis Campaign (Dellinger, et al., 2008) has produced international guidelines for the management of severe sepsis, including severe neutropenic sepsis. It recommends early investigations such as blood cultures and serum lactate, early administration of antibiotics (within 30 minutes), and goal directed resuscitation.
A number of risk scores which have influenced some current guidelines have come into use over the past few years. These include scores to identify those patients at both high and low risk of severe sepsis.
The Modified Early Warning Score (MEWS) (Subbe, et al., 2001) has been validated to identify seriously unwell adult patients within general medical wards rather than those with neutropenic sepsis, but it and similar scoring systems are in widespread use.
There are several specific risk scores for neutropenic sepsis which have the aim of identifying those patients at low risk of developing severe sepsis, meaning that less aggressive treatment than has been “traditional” may be appropriate. These cover both adults (Klastersky, et al., 2000) and children (Alexander, et al., 2002).
The details surrounding the treatment and prevention of neutropenic sepsis in published literature vary greatly. There is also no universally agreed definition of “neutropenia” and “sepsis” in this context amongst published literature (Clarke, et al., 2011).
1.2. The epidemiology of neutropenic sepsis in England and Wales
1.2.1. Incidence of neutropenic sepsis
The incidence of neutropenic sepsis in England and Wales is difficult to determine with any degree of certainty, because of variations in definition of neutropenic sepsis and lack of a consistent code used on NHS clinical coding databases.
Local audits and service reviews have addressed the subject of neutropenic sepsis and assessed the impact of the condition on individual hospitals, cancer networks and regions. These have not been nationally coordinated, used different methodologies/criteria for diagnosing neutropenic sepsis and covered differing clinical environments - from a single ward to an entire cancer network; nevertheless they do provide useful baseline information on the burden of the condition on healthcare (Table 1.1).
These surveys show that busier specialist units admit over 20 patients a month with neutropenic sepsis, while the burden on general hospitals is considerably less, approximately three patients per month. These rates will vary hugely depending on population size, tumour types treated locally, chemotherapy regimens used and local demographics.
Consideration should be given to performing a national prospective audit to capture all incidences of neutropenic sepsis and identify the burden of disease in the UK.
1.2.2. Mortality from neutropenic sepsis
The most important adverse outcome from an episode of neutropenic sepsis is the death of the patient. As part of this report, a study has been undertaken to assess the reported death rates from neutropenic sepsis over the past 10 years.
Methods
On the death of a patient, information from the Medical Certificate of Cause of Death is coded and recorded by the Office of National Statistics (ONS). A search of the ONS database between 2001 and 2010 was undertaken to identify patients (paediatric and adult) coded as having died with an underlying cancer diagnosis where both an infection and neutropenia were also reported on the death certificate. This means that “neutropenic sepsis”, “febrile neutropenia” and “neutropenia and pneumonia” would all have been captured. The search is performed using ICD10 codes rather than plain text (meaning incidences where neutropenic sepsis was implied on the death certificate but not coded as such may not have been captured). The numbers of patients recorded as having died from neutropenic sepsis was also compared to the number of cancer diagnoses in the same year in England (Office of National Statistics) and Wales (Wales Health Statistics). A summary of the ICD10 codes used in this search is listed in Appendix 1 of the full needs assessment report.
Results
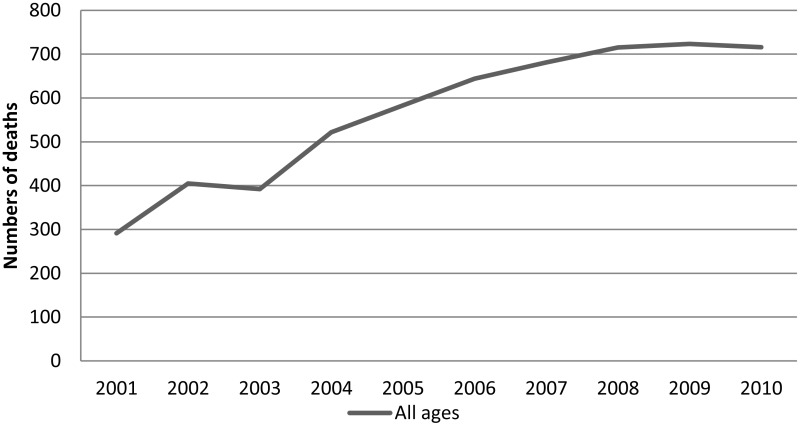
The total number of deaths from neutropenic sepsis has more than doubled over the period 2001 to 2010 (Figure 1.1).
There is a significant positive relationship between the year and total number of neutropenic sepsis deaths (p<0.001). Fitting fractional polynomials with the Multivariable Fractional Polynomials (MFP) package reported the best fit was achieved from a simple linear form.
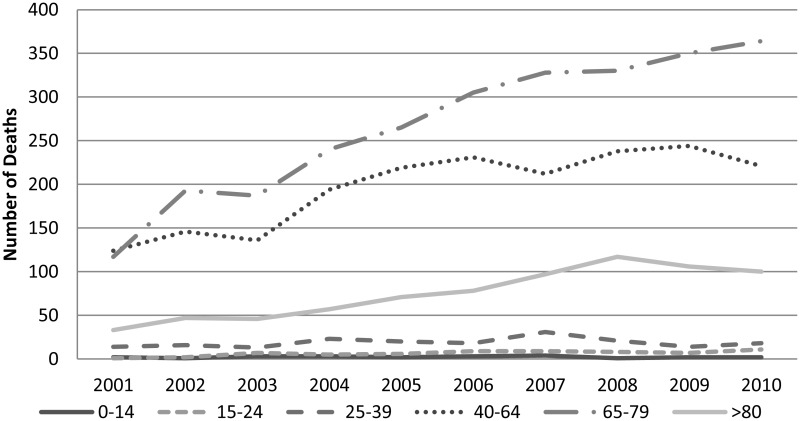
The age range 65 to 79 contains the majority of deaths. The death rate for younger patients appears to have remained fairly static over the years, although there has been an increase (Figure 1.2). The rate of this increase has been assessed and has been found to be the same over all the age ranges examined.
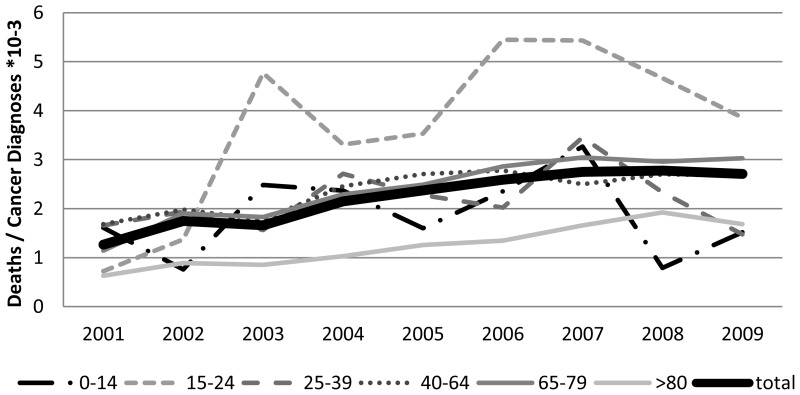
The number of deaths from neutropenic sepsis each year from 2000 to 2009 as a proportion of the annual total of cancer diagnoses (not including non-melanoma skin cancer) in each age group has been examined. Relative to the increased numbers of cancer diagnoses, the proportion of deaths due to neutropenic sepsis continues to rise for all groups. The rate of increase of neutropenic sepsis deaths is significantly higher for the 15-24 year old age group, and significantly lower for the >80 age group (Figure 1.3).
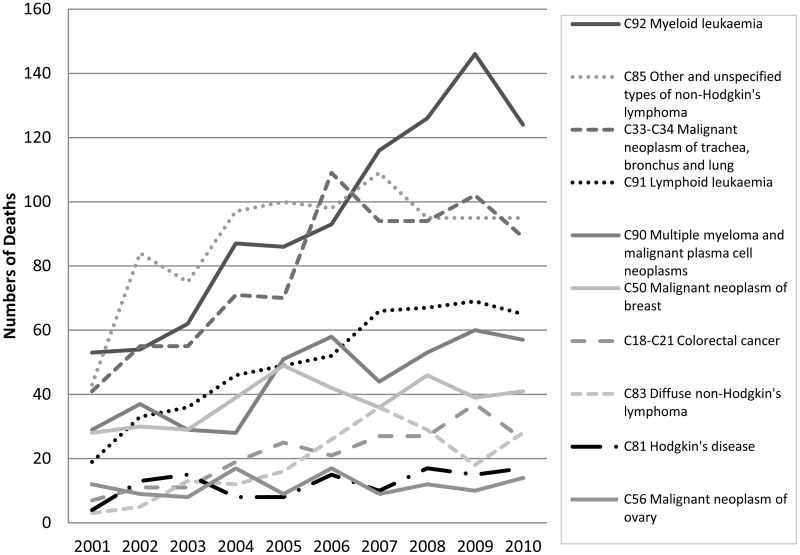
The 10 most common cancers where death involved neutropenic sepsis are shown in Figure 1.4.
Conclusions
The numbers of neutropenic sepsis deaths recorded by the ONS has more than doubled in 10 years, and there are now approximately two deaths each day in England and Wales from this complication of anticancer treatment.
There are several possible explanations for the increase in death rates. The numbers of cancers diagnosed each year is increasing, but as a proportion of those, the relative rate of neutropenic sepsis deaths also continues to rise. The NCAG report (NCAG 2009) stated that 60% more chemotherapy was given in 2006 than 2002. If this rise has continued, this alone is likely to be responsible for the increase in neutropenic sepsis deaths. Increasing intensity of chemotherapy regimens may be having an effect. It is also possible that more patients who previously might have been thought to have been too high risk for treatment are being given chemotherapy, and the NCEPOD report (NCEPOD, 2008) highlighted that selecting less fit patients for chemotherapy risks a higher rate of fatal complications, including neutropenic sepsis.
Patients aged 15 to 24 have a significantly higher risk of dying of neutropenic sepsis. It has been documented for many conditions that teenagers and young adults are less compliant with medical treatment and advice than older adults. This has certainly been seen for epilepsy (Asadi-Pooya, 2005) and diabetes (Cramer, 2004) amongst others, and is likely to impact on chemotherapy compliance with medical advice regarding neutropenic sepsis too (Gesundheit, et al., 2007). This, combined with the higher intensity of many of the chemotherapy regimens given to patients with cancer in this age group is likely to explain this finding.
Patients with a cancer diagnosis aged 80 or more have a significantly lower risk of dying of neutropenic sepsis. While there are still a large number of cancers being diagnosed in this group, considerably fewer patients are fit enough to receive chemotherapy, thus reducing the overall risk of neutropenic sepsis.
The most common underlying cancer diagnoses for patients dying of neutropenic sepsis are haematological malignancies, which have a relatively high rate of neutropenic sepsis, and the common solid tumours affecting adults.
It is well documented that the accuracy of death certificate completion has been poor (Swift and West 2002), and there have been recent drives to improve the quality and accuracy. Potentially, the increase in reported deaths may be due, at least in part, to increased accuracy of death certificate completion. There are currently pilot programs introducing a medical examiner role with the aim of introducing this system nationally by 2013. This may further improve the quality of the documentation.
It is unknown whether patients had a death certificate completed implying neutropenic sepsis which was not coded as such on the ONS database. Potentially, the increased death rate from neutropenic sepsis may in part be demonstrating an improvement in ONS coding accuracy, but there is no evidence either to support or refute this. Unfortunately, it was not possible to investigate this in more detail.
1.2.3. Influence of chemotherapy regimen on neutropenic sepsis
The risk of a patient developing neutropenic sepsis varies greatly according to the treatment regimen and, with certain regimens, whether prophylaxis has been given (Martin, et al., 2006). Risk factors for neutropenic sepsis can include advanced age, poor performance status, poor nutritional status, underlying haematological malignancy and intensity of chemotherapy (Lyman, 2005).
In 2006, as part of an American Society of Clinical Oncology (ASCO) guideline document, a review was performed of the published likelihood of the occurrence of neutropenic sepsis with various cytotoxic chemotherapy regimens thought to be of intermediate or high risk. In 2010 the European Organisation for the Research and Treatment of Cancer (EORTC) published a similar document (Aapro, et al., 2011) and also repeated the review. A selection of the more commonly used regimens to treat adult cancers in the UK is included in Table 1.2.
1.3. Current service provision for neutropenic sepsis in England and Wales
1.3.1. Methods
In order to determine the current practice concerning the prevention and treatment of neutropenic sepsis a questionnaire was distributed via the cancer networks to all acute trusts in England and Wales. A copy of the questionnaire can be found in the full needs assessment report. It was requested that this questionnaire be completed by a senior clinician (doctor or nurse) from any institution which may have to assess or treat a patient at risk of neutropenic sepsis. Several supporting documents were also requested, including any neutropenic sepsis, GCSF or relevant antibiotic policy documents, patient information, audits involving neutropenic sepsis and teaching materials. Where an institution had more than one neutropenic sepsis policy (it was recognised that policies for paediatrics, solid adult tumours and adult haemato-oncology could be different), it was requested that one questionnaire be completed for each policy, meaning some institutions were expected to return up to three questionnaires. The questionnaire covered all the main areas set out in the scope of the neutropenic sepsis guideline.
Where a questionnaire entry appeared to be incorrect or included a typographical error, any submitted documentation such as local neutropenic sepsis protocols was analysed and if necessary a correction was made. The range and scope of these questionnaire responses was described qualitatively or quantitatively as appropriate.
1.3.2. Results
Demographics
A total of 80 valid questionnaires were returned. 51 centres returned a single questionnaire, 11 returned two, 1 returned three and 1 returned four (as there was a separate policy covering lung cancer in this centre). The geographical distribution included representation from all areas of England and Wales. As the questionnaire was distributed via the cancer networks to the cancer leads for each hospital rather then directly to the trusts, it was not possible to determine a response rate.
These 80 questionnaires represented:
- 53 adult solid tumour policies
- 1 stand-alone centre
- 23 cancer centres within an acute trust
- 29 cancer units
- 44 haematology policies (Matthey, et al., 2009)
- 15 level 1
- 19 level 2
- 10 level 3&4 (including two level 4 units)
- 30 paediatric oncology policies
- 7 primary treatment centres
- 9 level 1 shared care units
- 4 level 2 shared care units
- 5 level 3 shared care units
- 5 paediatric departments without oncology
Definition of neutropenic sepsis
Temperature criteria
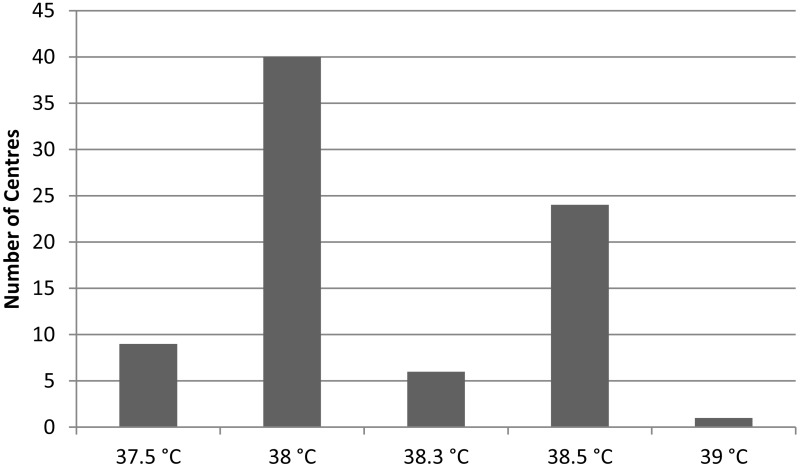
All centres had a single temperature above which the patient is considered to be at risk of neutropenic sepsis. The range of single readings varied from 37.5°C to 39°C (Figure 1.5).
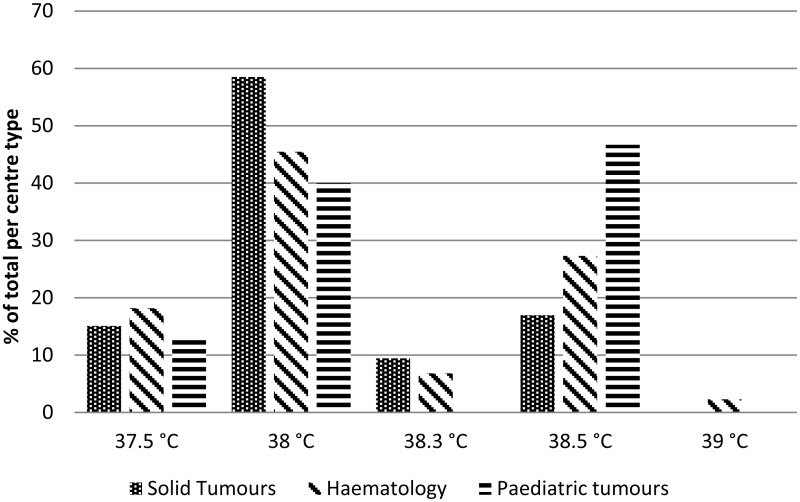
When split into paediatrics, adult solid tumours and adult haematology, the most common single temperature used for adults is 38°C and for children is 38.5°C (Figure 1.6).
In 36 (45%) of protocols, two temperature readings recorded over a period of time of a slightly lower grade fever than the single reading described above would trigger a potential “neutropenic sepsis” diagnosis. Of these, 20 (56%) listed two readings of 38°C over one hour. There were nine different criteria listed in total ranging from two temperatures of 37.5°C in 2 hours (adult and paediatric) to two readings of 38° over 4 hours (all paediatric). 19 (24%) of protocols included a minimum temperature for defining potential neutropenic sepsis.
Neutrophil criteria
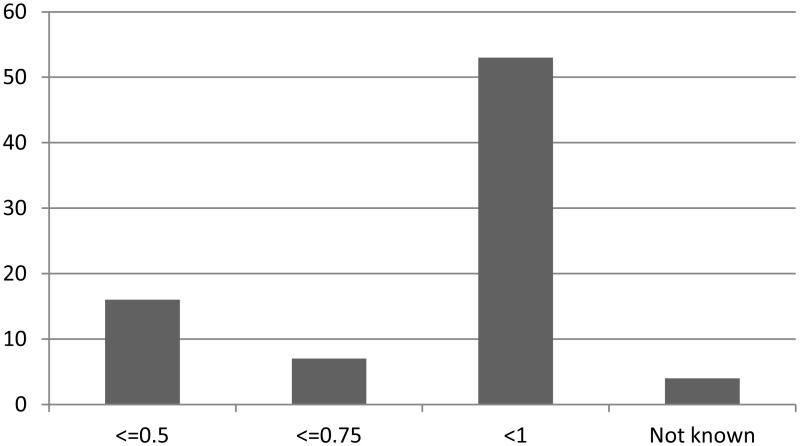
As with temperature criteria, the neutrophil count below which neutropenic sepsis was diagnosed varied between protocols (Figure 1.7).
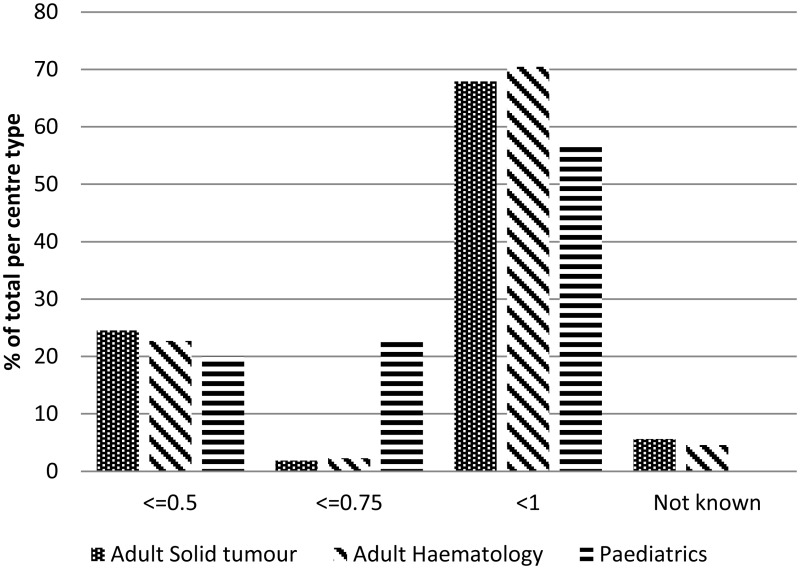
There appeared to be little difference between paediatric, adult solid tumour and adult haematology criteria for neutropenia (Figure 1.8).
Other criteria
The majority of protocols stated that if a patient was systemically unwell or shocked they would be treated as potentially having neutropenic sepsis regardless of the temperature. For the protocols where this was not explicitly stated, none suggested that a normal temperature excluded the diagnosis of neutropenic sepsis.
Prevention of neutropenic sepsis in adults and children
The two methods of prophylaxis against neutropenic sepsis covered by the guideline scope are antibiotics and GCSF.
Prophylactic antibiotics – primary prophylaxis
Primary antibiotic prophylaxis was reported as never used in 18 (23%) centres, was given for all regimens in 3 (4%) centres, and there were widely varying indications in the remaining 73%. The latter group were generally “high risk” regimens, including acute leukaemia, lung regimens, and high risk breast cancer regimens. Many of these centres gave antibiotic prophylaxis on cycle 1 alone.
There was no clear difference in the pattern of usage of prophylactic antibiotics between paediatric, adult solid tumour and adult haematology centres. The choice of prophylactic antibiotic was known for 35 policies. 77% used ciprofloxacin and 23% used levofloxacin.
Prophylactic antibiotics – secondary prophylaxis
Following an episode of neutropenic sepsis, secondary prophylactic antibiotic use was reported as never used in 31 (39%) policies, and used universally in 12 (15%). Where specified, ciprofloxacin was the commonest choice of antibiotic.
Prophylactic growth factors – primary prophylaxis
It was reported that growth factors (G-CSF) were never used in 4 (5%) protocols (including adult solid tumour, adult haematology and paediatrics) and were used in all regimes by 3 (4%). For the remainder, indications were very varied, and included “high risk” regimens in 39 (49%) protocols and only “high risk” regimens which were potentially curative in 8 (10%) protocols. Further criteria (for the remaining 32%) included a high risk of complications due to comorbidities, age, or regimen, or subjective criteria, for example “consultant decision”.
Where used for primary prophylaxis, G-CSF (as opposed to GM-CSF) was always prescribed. Around 80% of protocols for primary G-CSF prophylaxis used a once daily preparation and 20% used a long acting (pegylated) preparation for the majority of their regimens.
Prophylactic growth factors – secondary prophylaxis
Growth factors were used for secondary prophylaxis following an episode of neutropenic sepsis in 24 (30%) of centres for all further cycles, never used in 2 (3%) centres, and variably in the remainder. Most of the G-CSF used for this indication was given as a once daily rather than pegylated preparation.
Patient education
Written information
Of the 79 eligible centres (one was from an institute which did not deliver chemotherapy), 3 (4%) respondents stated their centres did not give written information which included information about neutropenic sepsis prior to chemotherapy. 57 (72%) gave written information at the initial visit, and the remainder gave the information at a subsequent clinic visit or just prior to chemotherapy. 51 (65%) routinely gave written information during more than one meeting.
Examples of written information given to patients ranged from a 76 page patient held record book covering all aspects of chemotherapy to single sided sheets reminding patients about neutropenic sepsis. The emphasis on neutropenic sepsis in the written information varied between it being the sole topic covered or it being discussed as part of a more general information resource, with no more emphasis on neutropenic sepsis than other chemotherapy toxicities. 29 (81%) information leaflets included advice concerning specific temperatures. 30 (83%) included a telephone number to call for advice.
Verbal information before chemotherapy
All centres where chemotherapy was administered reported that verbal information concerning neutropenic sepsis was routinely given prior to chemotherapy. 38 (48%) respondents reported their centres used a checklist for this.
Chemotherapy alert cards
62 (78%) respondents reported their centre provided a card or letter designed to be carried at all times while on chemotherapy. Examples contained either information for the patient, management advice to healthcare professionals or both. The information could include patient name and hospital number, the chemotherapy regimen, dates of delivery, symptoms of neutropenic sepsis, contact telephone numbers and specific advice to healthcare professionals on the treatment of neutropenic sepsis. While the majority were credit card sized, some were larger (still pocket sized) and there were a small number of examples of A4 sized letters.
Criteria for referral to secondary or tertiary care
Many protocols specified that advice should be sought if the patient was feeling generally unwell, experiencing rigors or had other concerns. Specific information about fever or hypothermia was given in most protocols. 54 (71%) protocols specified the same criteria as for diagnosing neutropenic sepsis in their centre, and 21 (27%) used a lower temperature to trigger a referral. 34 (44%) protocols also included instructions that the patient seek help if they developed a low temperature.
No policy mandated that patients had to have a certain temperature before seeking assistance.
Immediate management of neutropenic sepsis in adults and children
Initial antibiotic timing
76 (95%) respondents reported antibiotics were routinely given to patients presenting with suspected neutropenic sepsis before the full blood count was known. Of these, 57 (75%) would recommend antibiotics were started in all patients, and the remainder would perform a risk assessment (using a risk stratification tool such as the MASCC criteria (Kern, 2006) or clinical judgement.
75 (94%) respondents stated a “door to needle” time target was in place, and times were submitted for 73. (Table 1.3).
Several audits were submitted where “door to needle” time was evaluated. These tended to show that the “door to needle” time targets were initially poorly met, but improved on re-audit.
Initial empiric intravenous antibiotic choice (where oral antibiotics are not being considered)
Initial empiric intravenous antibiotic choice in patients not allergic to penicillin varied (Table 1.4). 27 (36%) use a single antibiotic while 48 (64%) used two or more antibiotics as their standard treatment.
The pattern of antibiotic use was generally the same in adult haematology, adult solid tumour and paediatric centres.
17 (21%) protocols used a risk assessment to identify those patients at higher risk of severe sepsis. 10 of these added gentamicin to the previous “standard” regimen and the 7 others changed to a completely different antibiotic regimen.
In patients with a central venous catheter, a different antibiotic regimen was recommended by 12 (15%) protocols; 9 added vancomycin and 3 added teicoplanin when a line infection was not suspected. Where infection was suspected 60 (75%) centres reported a specific policy; 33 added teicoplanin and 27 added vancomycin.
For a reported history of penicillin allergy but perceived low risk of anaphylaxis or angio- oedema, 64 (80%) protocols included a beta lactam-containing antibiotic such as ceftazadime or meropenem, while 12 (15%) policies contained no beta lactam antibiotics. For patients at high risk of penicillin related anaphylaxis, 28 (35%) respondents to the questionnaire quoted a regimen including a beta-lactam containing drug (mainly meropenem).
No centres in this study reported delivering first line intravenous antibiotics for neutropenic sepsis in an ambulatory care setting.
Empirical oral antibiotics
Empirical oral antibiotics were given to lower risk patients in 23 (29%) protocols. Most centres using such a policy discharged patients immediately, with the minority observing for up to 24 hours or more.
Where a specific risk scoring system was used, the MASCC score (Kern, 2006) was most frequently quoted. Some high risk tumour types such as acute leukaemia were specifically excluded from receiving oral antibiotics in most of these regimens. Some centres only used such an oral antibiotic policy for palliative chemotherapy regimens. Where the patient had been on prophylactic oral antibiotics or G-CSF they were generally excluded from receiving oral antibiotics to treat neutropenic sepsis.
Ciprofloxacin and co-amoxiclav were the most common antibiotic choices. Clindamycin was most commonly used if the patient was allergic to penicillin.
On-going management of neutropenic sepsis
Two situations were considered:
- Uncomplicated admission, where the patient's pyrexia settles
- Failure to respond to first line antibiotics
Uncomplicated admission
Approximately two-thirds of centres of all types routinely switched from intravenous to oral antibiotics before discharge. Criteria for switching varied, including: after a set number of days (from 1 to 5); when the patient was apyrexial and had a rising neutrophil count; when the patient had been apyrexial for a given length of time, regardless of neutrophil count.
The majority of centres observed the patient 24 hours after stopping intravenous antibiotics before discharge. This was the case both if they had been changed to oral antibiotics or when antibiotics had been stopped completely.
Failure to respond to first line antibiotics
54 (68%) centres routinely changed the antibiotic regimen after 48 hours without improvement. 16 (20%) centres changed after 24 hours, and 10 (12%) considered changing after 3 or 4 days.
Documentation concerning neutropenic sepsis
All but one centre had a written neutropenic sepsis policy, and all but two had a specific antibiotic policy for neutropenic sepsis.
Staff training
Staff training varied across trusts and disciplines. The majority of respondents reported some form of training for junior doctors and nurses, and provided this information through direct education and provision of internet and various written information sources.
1.4. Summary
Neutropenic sepsis is common, resulting in hundreds of hospital admissions every month and potentially causing the deaths of over 1 in 500 people diagnosed with cancer. There is evidence that the number of deaths from neutropenic sepsis is increasing at a faster rate than the number of cancers being diagnosed. The most likely explanation for this is the increase in the amount of chemotherapy administered in recent years (NCAG 2009). If each chemotherapy cycle prescribed carries a risk of neutropenic sepsis, it is highly likely that the incidence, and therefore the rare event of a death from neutropenic sepsis will have increased too. Despite the very small numbers, there is a significantly greater risk of death from neutropenic sepsis in patients aged 15-24 years old.
Unfortunately it has not been possible to determine the overall burden of neutropenic sepsis on the NHS in England and Wales, largely because the GDG did not feel the accuracy of coding for neutropenic sepsis in clinical coding databases could be relied on at present, although it is recognised that efforts are being made to improve this.
Despite the significance of neutropenic sepsis and the national recognition of the importance of the condition, there is surprisingly little agreement throughout England and Wales regarding its definition, prevention, diagnosis and treatment. This echoes the findings of recent studies covering haemato-oncology (Clarke, et al., 2011) and paediatric oncology (Phillips, et.,2007).
- Definitions of neutropenia ranged from a neutrophil count of 0.5×109/L to 1.0 ×109/L. A temperature at which a patient would be treated empirically varied from 37.5 °C to 39°C, with the majority using 38°C.
- Policies concerning prophylaxis with G-CSF and/or antibiotics were very varied for both primary and secondary prophylaxis.
- Almost all centres had a “door to needle” time of one hour or less, when giving intravenous antibiotics to a patient suspected of having neutropenic sepsis, as mandated in the recent NCAG report (NCAG 2009). The antibiotics given varied considerably, but the majority of centres used either gentamicin and piperacillin / tazobactam or piperacillin/tazobactam alone.
- Approximately a third of centres had a policy where lower risk patients are given oral instead of intravenous antibiotics. Most patients were discharged immediately if started on this pathway.
- It was almost universal that patients received written and verbal information about neutropenic sepsis before chemotherapy was administered, or occasionally (in paediatric settings) before discharge following in-patient chemotherapy.
- Almost all centres had a written neutropenic sepsis policy, communicated to staff via training, posters, hospital intranets and handbooks.
A major methodological challenge in assessing the rate of neutropenic sepsis, infections and death in England and Wales was the variable quality and lack of consistency of death certification and clinical coding. This makes assessing the impact of neutropenic sepsis on patients, carers and the health service as a whole very difficult and probably impossible. While neutropenic sepsis is a complication of anticancer treatment rather than a diagnosis in itself, consideration should be given to assigning it a unique ICD10 code to better define the effect of this complication.
The dramatic variations seen here concerning the definitions, prevention and treatment of neutropenic sepsis highlight the need for an evidence based guideline to guide and unify UK practice.
Research Recommendation
- A prospective national cohort study to assess the incidence of suspected and proven neutropenic sepsis in patients having anticancer treatment.
Linking Evidence to Recommendations
The GDG noted that during the needs assessment work it had been difficult to assess the incidence and burden of treating neutropenic sepsis. They agreed that further research needs to be undertaken to assess the incidence of suspected and proven neutropenic sepsis.
References
- Aapro MS, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. European journal of cancer. 2011;47(1):8–32. [PubMed: 21095116]
- Alexander SW, Wade KC, Hibberd PL, Parsons DPH, Parsons SK. Evaluation of Risk Prediction Criteria for Episodes of Febrile Neutropenia in Children With Cancer. Journal of Paediatric Haematology/Oncology. 2002;24(1):38–42. [PubMed: 11902738]
- Asadi-Pooya AA. Drug compliance of children and adolescents with epilepsy. Seizure: the journal of the British Epilepsy Association. 2005;14(6):393–5. [PubMed: 15978849]
- Bhatt V, Saleem A. Review: Drug-induced neutropenia--pathophysiology, clinical features, and management. Annals of clinical and laboratory science. 2004;34(2):131–7. [PubMed: 15228223]
- Cardenal F, et al. Randomized phase III study of gemcitabine-cisplatin versus etoposide- cisplatin in the treatment of locally advanced or metastatic non-small-cell lung cancer. Journal of clinical oncology official journal of the American Society of Clinical Oncology. 1999;17(1):12–8. [PubMed: 10458212]
- Clarke RT, Warnick J, Stretton K, Littlewood TJ. Improving the immediate management of neutropenic sepsis in the UK: lessons from a national audit. British journal of haematology. 2011;153(6):773–9. [PubMed: 21517822]
- Cramer JA. Adherence With Medications for Diabetes. Diabetes Care. 2004;27(2003):1218–1224. [PubMed: 15111553]
- Crawford J, et al. Myeloid growth factors. Journal of the National Comprehensive Cancer Network: JNCCN. 2011;9(8):914–32. [PubMed: 21900221]
- Cunningham D, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. The New England journal of medicine. 2010;362(9):858–9. [PubMed: 20200397]
- Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil kinetics in man. The Journal of clinical investigation. 1976 Sep;58(3):705–15. [PMC free article: PMC333229] [PubMed: 956397]
- Dellinger RP, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Critical Care Medicine. 2008;36(1):296–327. [PubMed: 18158437]
- Dommett R, et al. Successful introduction and audit of a step-down oral antibiotic strategy for low risk paediatric febrile neutropaenia in a UK, multicentre, shared care setting. European journal of cancer. 2009;45(16):2843–9. [PubMed: 19616427]
- Douillard JY, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–7. [PubMed: 10744089]
- Gesundheit B, Greenberg ML, Reuven OR, Koren G, Koren G. drug compliance by adolescent and young adult cancer Patients: challenges for the Physician. Springer; Cancer in Adolescents and Young adults. 2007:353–363. Book title.
- Head J, Archer C, Harper Wynee C, Sinha R, Ring A, Ring A, Ring A, Banner, Sutherland, Johnston S. Rates of neutropaenic sepsis with the use of adjuvant FEC100-Docetaxel (FEC100-T) chemotherapy in high-risk node-positive patients with early breast cancer; A UK perspective. 2008. poster abstract, http://www
.ncri.org.uk /ncriconference/2008abstracts /abstracts/B64.htm. - Herbst C, et al. Prophylactic antibiotics or G-CSF for the prevention of infections and improvement of survival in cancer patients undergoing chemotherapy ( Review ). Cochrane Collaboration. 2009 [PubMed: 19160320]
- Holmes FA. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Annals of Oncology. 2002;13(6):903–909. [PubMed: 12123336]
- Kern WV. Risk assessment and treatment of low-risk patients with febrile neutropenia. Clinical infectious diseases. 2006;42(4):533–40. [PubMed: 16421798]
- Klastersky J, et al. The Multinational Association for Supportive Care in Cancer risk index: A multinational scoring system for identifying low-risk febrile neutropenic cancer patients. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2000;18(16):3038–51. [PubMed: 10944139]
- Lyman GH, et al. Risk Models for Predicting Chemotherapy-Induced Neutropenia. The Oncologist. 2005;(10):427–437. [PubMed: 15967836]
- Matthey F, Parker A, Rule SAJ, Wimperis JZ. Facilities for the Treatment of Adults with Haematological Malignancies – ‘ Levels of Care ’ BCSH Haemato-Oncology Task Force 2009. 2009. http://www
.bcshguidelines .com/documents/levelsofcare_042010 .pdf . [PubMed: 20423565] - Martin M, et al. Adjuvant Docetaxel for Node-Positive Breast Cancer. New England Journal of Medicine. 2005;352(22):2302–2313. [PubMed: 15930421]
- Martín M, et al. Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2006;17(8):1205–12. [PubMed: 16766587]
- NCEPOD. For better or worse: A review of the care of patients who died within 30 days of receiving systemic anticancer therapy. 2008. http://www
.ncepod.org.uk/2008sact.htm . - Nathan C. Neutrophils and immunity: challenges and opportunities. Nature reviews Immunology. 2006;6(3):173–82. [PubMed: 16498448]
- National Chemotherapy Advisory Group. Chemotherapy Services in England: Ensuring quality and safety a report from the National Chemotherapy Advisory Group. 2009. http://ncat
.nhs.uk/sites /default/files/NCAG%20Report.pd . - North Wales Cancer Network Audit of neutropenic sepsis in chemotherapy patients from North Wales. http://www
.wales.nhs .uk/sites3/docmetadata .cfm?orgid=456&id=123172 . - Office of National Statistics. http://www
.statistics .gov.uk/hub/index.html . - Okera M, et al. A prospective study of chemotherapy-induced febrile neutropenia in the South West London Cancer Network. Interpretation of study results in light of NCAG/NCEPOD findings. British journal of cancer. 2011;104(3):407–12. [PMC free article: PMC3049562] [PubMed: 21179036]
- Phillips B, Selwood K, Lane SM, Skinner B, Gibson F, Chisholm JC. Variation in policies for the management of febrile neutropenia in United Kingdom Children's Cancer Study Group centres. Archives of disease in childhood. 2007;92(6):495–8. [PMC free article: PMC2066132] [PubMed: 17284481]
- Pillay J, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116(4):625–7. [PubMed: 20410504]
- Richardson S, Pallot D, Hughes T, Littlewood T. Improving management of neutropenic sepsis in the emergency department. British journal of haematology. 2009;144(4):617–8. [PubMed: 19016714]
- Rothenberg M, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003;21(11):2059–69. [PubMed: 12775730]
- Schwenkglenks M, et al. Hodgkins lymphoma treatment with ABVD in the US and the EU: neutropenia occurrence and impaired chemotherapy delivery. Journal of Hematology & Oncology. 2010;3:27. [PMC free article: PMC2933589] [PubMed: 20723212]
- Services H, Wilmot JP. Risk models for predicting chemotherapy induced neutropenic sepsis. New York: 2005. pp. 427–437.
- Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. Critical Care Medicine. 2001:521–526. [PubMed: 11588210]
- Swift B, West K. Death certification: an audit of practice entering the 21st century. Journal of clinical pathology. 2002;55(4):275–9. [PMC free article: PMC1769637] [PubMed: 11919211]
- Teoh RSEM, Huddart R, Dearnley D, Horwich A, Van As N. Incidence of neutropenia and neutropenic sepsis in patients with testicular cancer receiving chemotherapy. 2006. http://www
.ncri.org.uk /ncriconference/abstract /pdf/pdfs/NCRI2006_0598.pdf . - Vakkalanka B, Link BK. Neutropenia and Neutropenic Complications in ABVD Chemotherapy for Hodgkin Lymphoma. Advances in hematology. 2011;2011 Article ID 656013. [PMC free article: PMC3112508] [PubMed: 21687649]
- Van Vliet MC, Potting MJ, Sturm PDJ, Donnelly JP, Blijlevens NMA. How prompt is prompt in daily practice? Earlier initiation of empirical antibacterial therapy for the febrile neutropenic patient. European journal of cancer care. 2011;20(5):679–85. [PubMed: 21771130]
- Vermorken JB, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. The New England journal of medicine. 2007;357(17):1695–704. [PubMed: 17960012]
- Wales health statistics. http://wales
.gov.uk/topics /statistics/theme/health/?lang=en . - Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Laboratory investigation. 2000;80(5):617–53. [PubMed: 10830774]
Figures
Tables
Table 1.1Summary of audits and reviews of rates of neutropenic sepsis
| Time period | Number of cases | Audit description | Source |
|---|---|---|---|
| 05/2007 − 08/2007 | 71 admissions in 64 patients | Audit of all patients admitted with neutropenic sepsis to the seven hospitals of the South West London Cancer Network (population 1.4 million) | Okera, at al., 2011 |
| 2 months | 29 patients | Single institution audit at John Radcliffe Hospital, Oxford of patients admitted either to A&E or haematology. | Richardson, et al., 2009 |
| 1 year (2008) | 128 episodes in 119 patients | Single institution service improvement audit for an adult haematology department (no solid tumours) of episodes of neutropenic sepsis on the haematology ward. | Van Vliet, et al., 2011 |
| 1 year (1/4/04 to 31/3/05) | 762 episodes in 368 patients | 4 Paediatric Oncology Centres (averaging 74.7 episodes each) and 43 Paediatric Oncology Shared Care Units (averaging 13.5 episodes each) in London | Dommett, et al., 2009 |
| 1/1/2009 to 31/3/2009 | 32 episodes | 3 hospitals of the North Wales Cancer Network | North Wales Cancer Network Audit of neutropenic sepsis in chemotherapy patients from North Wales |
| 6 months | 22 patients admitted through A&E | Mainly haematology patients in an adult cancer unit/haemato-oncology unit. | Submitted from survey |
| January 2008 to April 2009 | 42 episodes | Audit of a North-London general hospital with a cancer unit and adult haemato-oncology unit using coding for neutropenia to select cases | Submitted from survey |
| 08/2010 to 10/2010 | 33 patients | Haematology and oncology unit in East London - two other audits from this hospital displayed similar results | Submitted from survey |
| 03/2011 to 06/2011 inclusive | 92 cases in 84 patients | Admissions to a Yorkshire Cancer centre for cancers treated there or in nearby cancer units (including some lymphoma but no other haemato-oncology) | Submitted from survey |
Table 1.2Risk of neutropenic sepsis from differing chemotherapy regimens
| Tumour site | Regimen | Likelihood of neutropenic sepsis (%) | Trial |
|---|---|---|---|
| Breast | TAC1 | 28.8 | Martin, et al., 2005 |
| FEC100-T2 | 25 | Head, et al., 2008 | |
| FAC3 | 4.4 | Martin, et al., 2005 | |
| Lung | Carboplatin / Etoposide | 10-20 | Crawford, et al., 2011 |
| Gemcitabine / Cisplatin | 7 | Cardenal, 1999 | |
| Colorectal | FOLFIRI4 | 11 | Douillard, et al., 2000 |
| FOLFOX45 | 6 | Rotheberg, et al., 2003 | |
| Gastric / Oesophageal | EOX6 | 10 | Cunningham, et al., 2010 |
| NHL | CHOP7 | 35 | Lyman, et al., 2003 |
| Hodgkin disease | ABVD8 | 2-12% | Vakkalanka, Link, 2011 |
| Germ cell | BEP9 (including CBOP-BEP)10 | 18 | Teoh, et al., 2006 |
| Head and neck | TPF11 | 9 | Vermorken, 2007 |
- 1
Docetaxel 75mg/m2, doxorubicin 50mg/m2, cyclophosphamide 500mg/m2 d1 of 21 day cycle
- 2
Fluorouracil 500mg/m2, epirubucin 100mg/m2, cyclophosphamide 500mg/m2, d1 of 21 day cycle for 3 cycles then docetaxel 100mg/m2 d1 of 21 day cycle for 3 cycles
- 3
Fluorouracil 500mg/m2, doxorubicin 50mg/m2, cyclophosphamide 500mg/m2 d1 of 21 day cycle
- 4
Either irinotecan 80mg/m2, fluorouracil infusion (24h) 2300mg/m2, calcium folinate 500mg/m2 d1 weekly OR irinotecan 180mg/m2, fluorouracil 400mg/m2 bolus and 600mg/m2 22 hour infusion and calcium folinate 500mg/m2 d1 of 14 day cycle
- 5
Oxaliplatin 85mg/m2 d1, leucovarin 200mg/m2, fluorouracil 400mg/m2 bolus and 600mg/m2 22 hour infusion d1 and 2 of 14
- 6
Epirubicin 50mg/m2, oxaliplatin 130mg/m2 and d1 capecitabine 625mg/m2 bd daily 21 day cycle
- 7
Cyclophosphamide 750mg/m2, doxorubicin 50mg/m2, vincristine 1.4mg/m2 d1 and prednisolone 100mg d1-5 of 21 day cycle
- 8
Doxorubicin 25mg/m2, bleomycin 10,000u, vinblastine 6mg/m2 and dacarbazine 375mg/m2 d1 and 15 of 28 day cycle
- 9
Bleomycin, etoposide and cisplatin (exact doses not specified from this source)
- 10
Bleomycin, etoposide, cisplatin, vincristine and carboplatin (exact dose and schedule not specified from this source)
- 11
Docetaxel 75mg/m2, Cisplatin 75 mg/m2, fluorouracil 750mg/m2, d1 of 21 day cycle
Table 1.3Door to needle times
| Door to needle time | Number of protocols |
|---|---|
| 30 minutes | 5 (7%) |
| 1 hour | 65 (89%) |
| 2 hours | 3 (4%) |
Table 1.4Antibiotic protocols
| Antibiotic regimen | Number of protocols |
|---|---|
| Piperacillin / tazobactam and gentamicin | 43 (57%) |
| Piperacillin / tazobactam monotherapy | 19 (25%) |
| Meropenem monotherapy | 8 (11%) |
| Piperacillin / tazobactam and amikacin | 3 (4%) |
| Ceftazadime and gentamicin | 1 (1%) |
| Ceftriaxone and gentamicin | 1 (1%) |